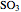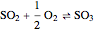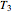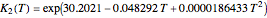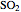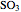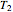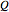The sulfur dioxide then undergoes a catalytic conversion into sulfur trioxide, 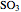 , in an adiabatic reactor:
, in an adiabatic reactor: 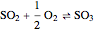 .
.
Sulfur trioxide is prepared on a massive scale as a precursor to sulfuric acid.
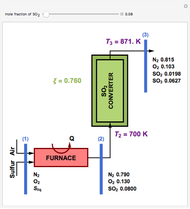
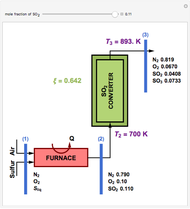
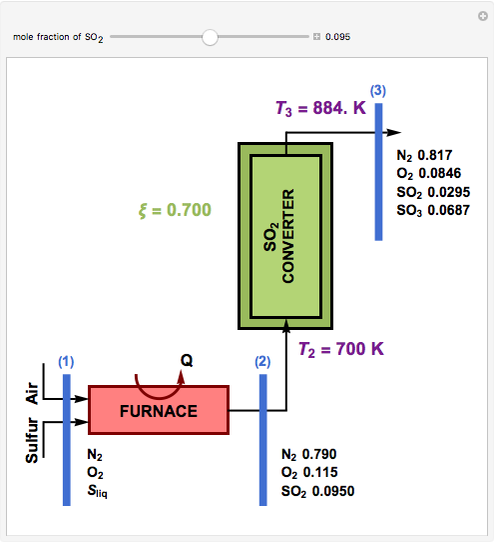
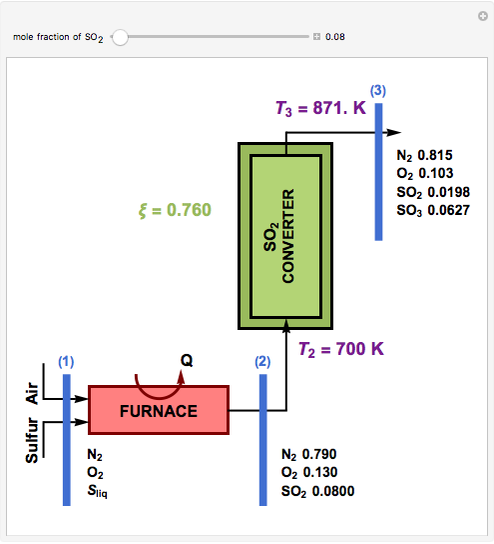
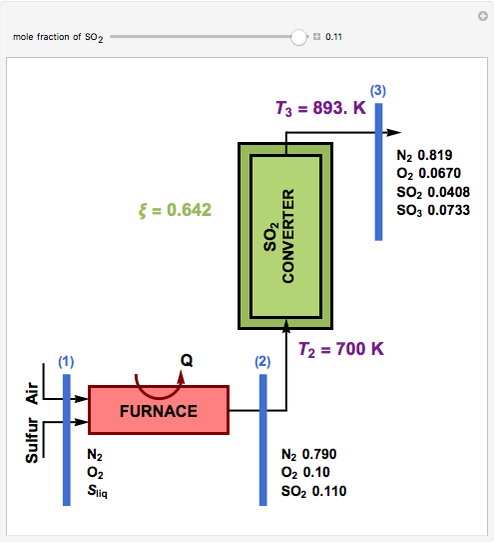
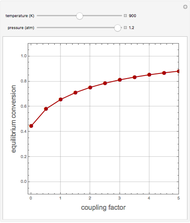
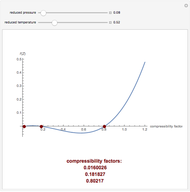
This Demonstration computes the extent of reaction,  , the exit temperature,
, the exit temperature, 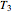 , and the composition of the effluent for the adiabatic converter used for
, and the composition of the effluent for the adiabatic converter used for 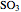 production. To do so, one has to write: (1) the chemical equilibrium using the equilibrium constant
production. To do so, one has to write: (1) the chemical equilibrium using the equilibrium constant 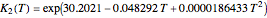 , obtained by fitting the free energy data versus temperature for
, obtained by fitting the free energy data versus temperature for 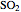 and
and 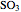 , and (2) the energy balance:
, and (2) the energy balance:  , which exploits the fact that the converter is adiabatic.
, which exploits the fact that the converter is adiabatic.
Finally, the temperature at the entrance of the converter is chosen equal to 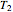 =700 Kelvin, a value that depends on the energy produced in the furnace,
=700 Kelvin, a value that depends on the energy produced in the furnace, 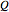 , used to generate live or superheated steam.
, used to generate live or superheated steam.
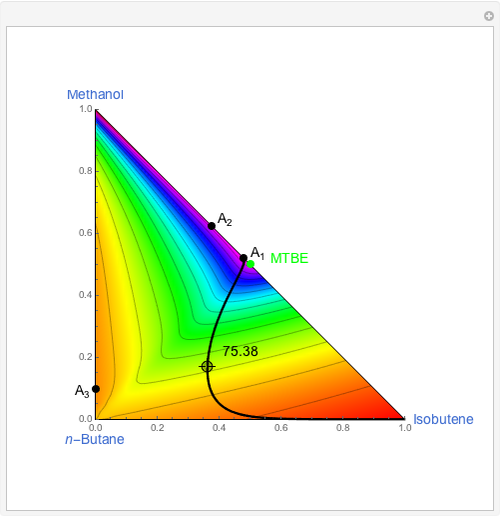
The user-specified values of the mole fraction of 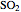 are in the range typically used in the industry.
are in the range typically used in the industry.
[less]

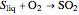 .
.