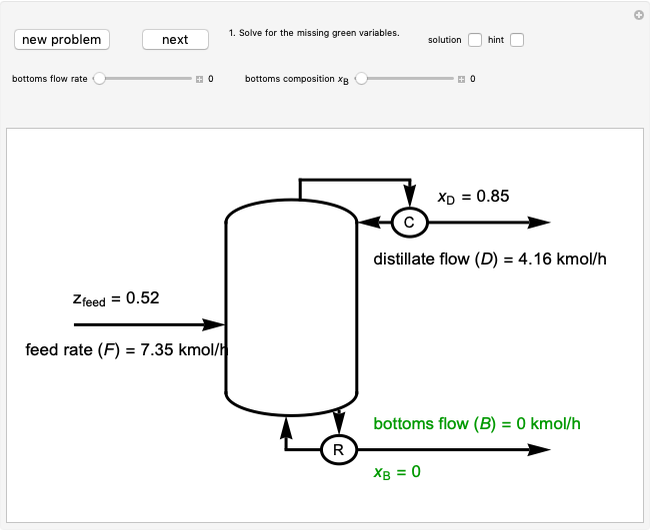
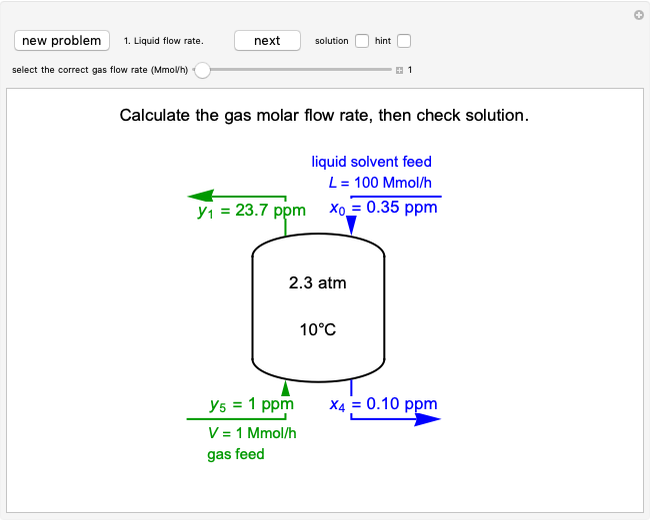
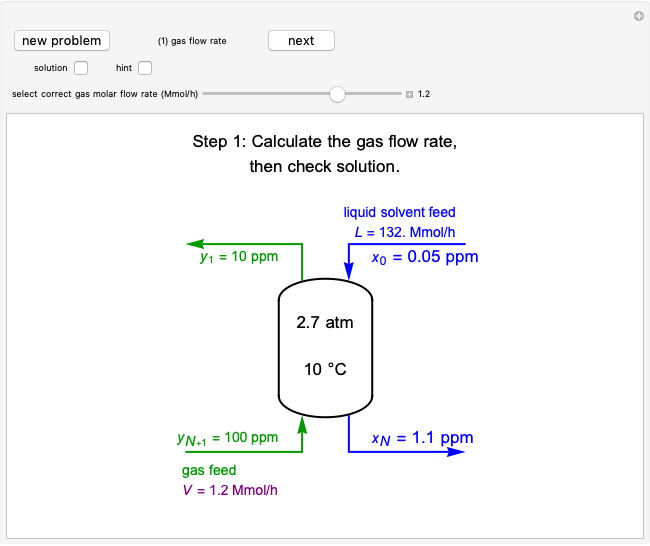
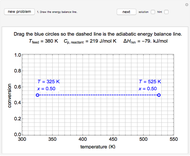
Adiabatic Flash Drum with Binary Liquid Feed

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
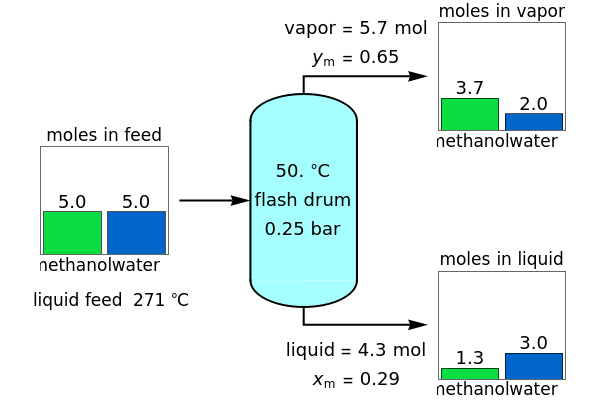
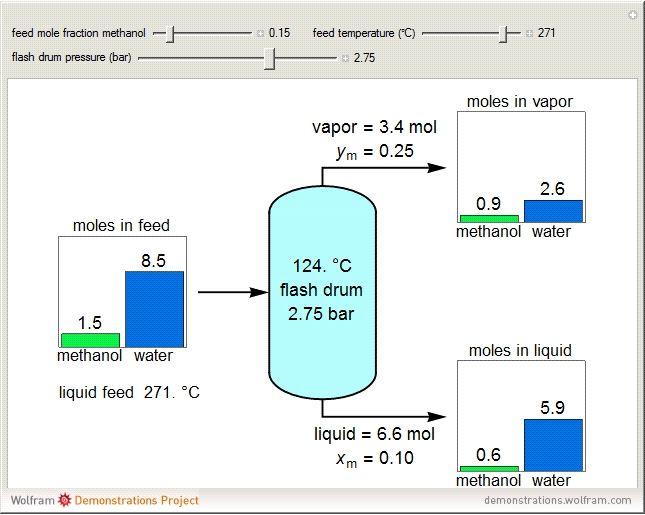
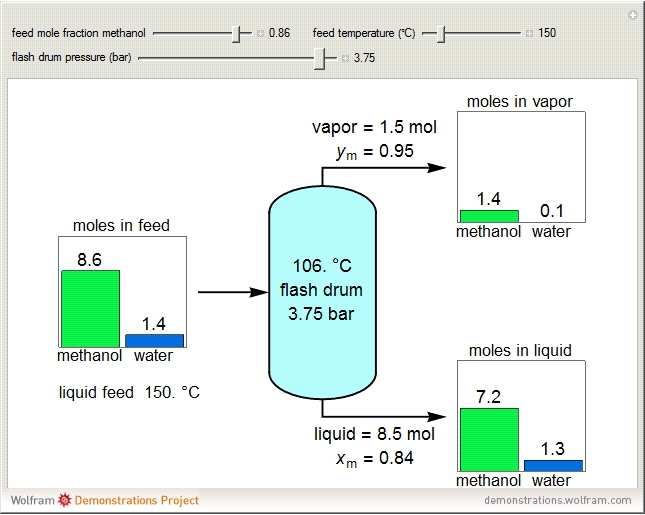
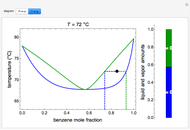
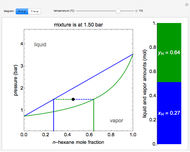
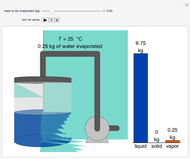
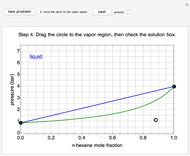
A high-pressure, hot, liquid mixture of methanol and water is fed into an adiabatic flash drum (or vapor-liquid separator). Because the flash drum pressure is below the bubble pressure, some of the liquid evaporates and the temperature decreases because energy is needed to evaporate the liquid. Thus, a vapor-liquid mixture in equilibrium exits the drum. You can vary the feed mole fraction of methanol, the feed temperature and flash drum pressure with sliders. This is a continuous process, but calculations are presented for 10 moles of feed. Material balances, an energy balance and Raoult's law for vapor-liquid equilibrium are used to determine the amounts of liquid and vapor exiting the drum and the mole fractions in each phase.
Contributed by: Derek Machalek and Rachael L. Baumann (June 2015)
Additional contributions by: John L. Falconer
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
An overall and component mole balance are performed:
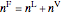 ,
,
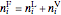 ,
,
where  is the number of moles, the superscripts
is the number of moles, the superscripts 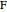 ,
,  and
and 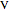 refer to the feed, liquid and vapor streams and the subscript
refer to the feed, liquid and vapor streams and the subscript 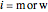 refers to a component (methanol or water).
refers to a component (methanol or water).
Overall and component energy balances with the reference state 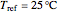 and
and 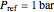 :
:
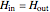 ,
,
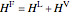 ,
,
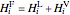 ,
,
where 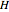 is enthalpy (kJ).
is enthalpy (kJ).
The enthalpies of each stream are calculated using heat capacities 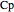 (kJ/(mol K)) and heat of vaporization
(kJ/(mol K)) and heat of vaporization 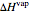 (kJ/mol):
(kJ/mol):
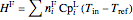 ,
,
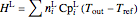 ,
,
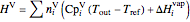 .
.
The flash drum has a single equilibrium stage, so the exiting liquid and vapor streams are at the same temperature, 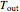 .
.
Saturation pressure 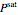 of the components in vapor-liquid equilibrium is calculated using the Antoine equation:
of the components in vapor-liquid equilibrium is calculated using the Antoine equation:
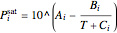 ,
,
where 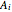 ,
, 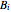 and
and 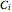 are Antoine constants for each component, and
are Antoine constants for each component, and 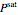 is in bar.
is in bar.
Raoult's law is used for the exit streams to find the vapor-liquid equilibrium compositions:
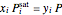 ,
,
where 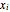 and
and 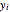 are the liquid and vapor mole fractions.
are the liquid and vapor mole fractions.
The sum of the mole fractions times their saturation pressures is the total pressure 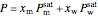 .
.
The screencast video at [1] explains how to use this Demonstration.
Reference
[1] Adiabatic Flash Drum with Binary Liquid Feed [Video]. (Dec 8, 2016) www.colorado.edu/learncheme/separations/AdiabaticFlash.html.
Permanent Citation