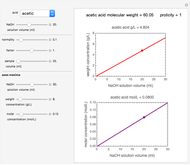
Arrhenius versus Exponential Model for Chemical Reactions

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
The temperature dependence of the rate of chemical reactions and biological processes is frequently described by the Arrhenius equation. This implies the existence of a log-linear relationship between the rate constant and the reciprocal of the absolute temperature, which has been used to calculate the "energy of activation". It is shown here that there are many conditions in which data generated with a simpler exponential model can be fitted with the Arrhenius equation and vice versa. This suggests that an Arrhenius plot's linearity is insufficient to establish that there is a temperature-independent "energy of activation" unless its existence is confirmed independently.
Contributed by: Mark D. Normand and Micha Peleg (July 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
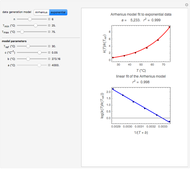
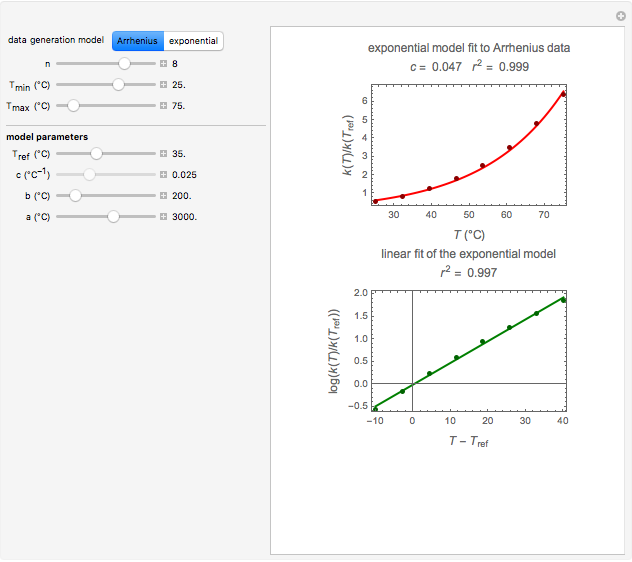
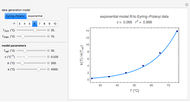
Snapshot 1: data generated with an Arrhenius-like equation where the absolute temperature reciprocal is replaced by 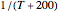 and then fitted with the exponential model
and then fitted with the exponential model
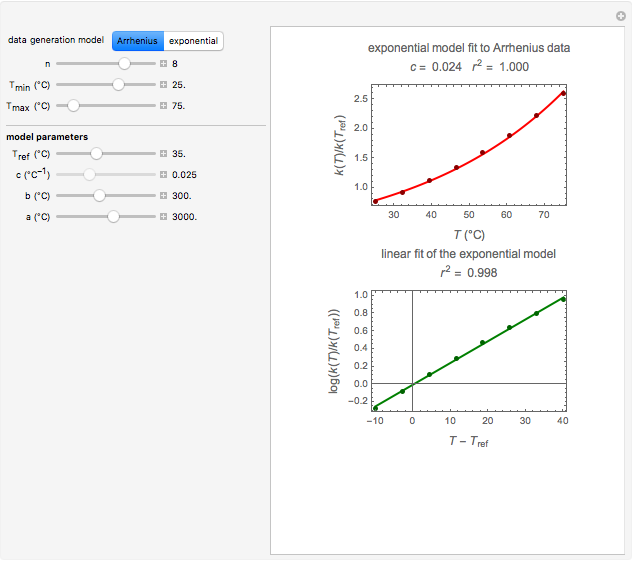
Snapshot 2: data generated with an Arrhenius-like equation where the absolute temperature reciprocal is replaced by 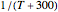 and then fitted with the exponential model
and then fitted with the exponential model
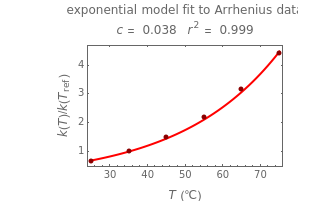
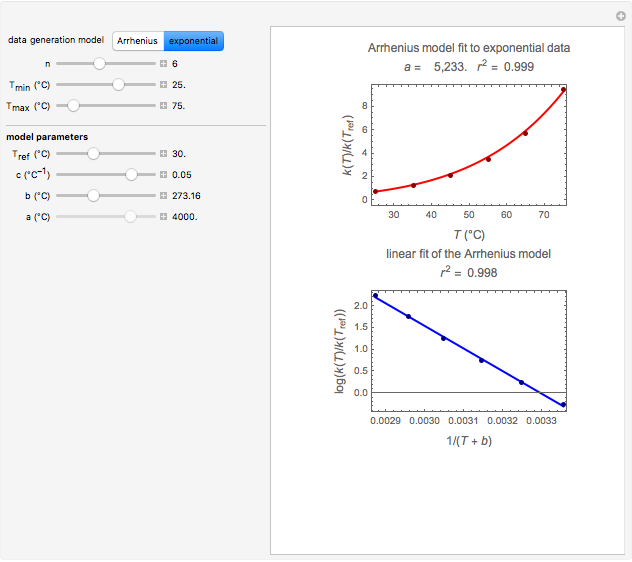
Snapshot 3: data generated with the exponential model and fitted with the standard Arrhenius equation (notice the linearity of the Arrhenius plot in blue)
Snapshot 4: data generated with the exponential model and fitted with an Arrhenius-like equation where the absolute temperature reciprocal is replaced by 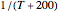
Snapshot 5: data generated with the exponential model and fitted with an Arrhenius-like equation where the absolute temperature reciprocal is replaced by 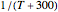
This Demonstration generates 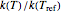 versus
versus 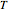 datasets using the Arrhenius or Arrhenius-like equations that are fitted with an exponential model. It also generates such datasets using the exponential model that are fitted by the Arrhenius or Arrhenius-like equations. The Arrhenius-like equation is presented as
datasets using the Arrhenius or Arrhenius-like equations that are fitted with an exponential model. It also generates such datasets using the exponential model that are fitted by the Arrhenius or Arrhenius-like equations. The Arrhenius-like equation is presented as 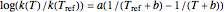 , where
, where 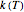 and
and 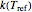 are the rates at temperature
are the rates at temperature 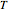 and the reference temperature,
and the reference temperature, 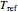 , respectively, both in °C, and
, respectively, both in °C, and  and
and  are constants having temperature dimension. Notice that setting
are constants having temperature dimension. Notice that setting  to 273.16 °C produces the standard Arrhenius equation. The exponential model equation is
to 273.16 °C produces the standard Arrhenius equation. The exponential model equation is 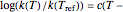
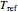 ) where
) where  is a constant having temperature reciprocal units (°
is a constant having temperature reciprocal units (°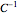 ).
).
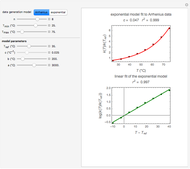
The user chooses the data generation model by clicking the setter bar. The data so generated will be fitted by the other model. The number of points,  , the plot's temperature range,
, the plot's temperature range, 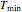 and
and 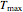 , the reference temperature,
, the reference temperature, 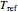 , and the generation or fit parameters
, and the generation or fit parameters  ,
,  , and
, and  , are entered with sliders.
, are entered with sliders.
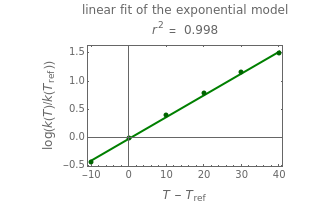
The display includes the generated 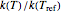 versus
versus 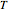 data and their fit by the opposite model (top plot) and the
data and their fit by the opposite model (top plot) and the 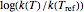 versus
versus 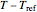 or
or 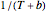 data fitted by the opposite model (bottom plot).
data fitted by the opposite model (bottom plot).
Notice that when  is large, the Arrhenius plot of the data generated by the exponential model (blue dots in bottom plot) is almost perfectly linear regardless of its absolute magnitude.
is large, the Arrhenius plot of the data generated by the exponential model (blue dots in bottom plot) is almost perfectly linear regardless of its absolute magnitude.
All curves generated by the Arrhenius equation change concavity at a high enough temperature. When the generated data has downward concavity at the chosen settings, the Demonstration forgoes the fitting by the exponential model and displays a message to that effect.
Permanent Citation