Balmer Series for the Bohr Atom

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
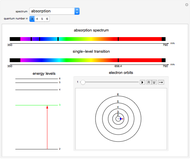
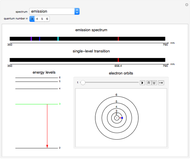
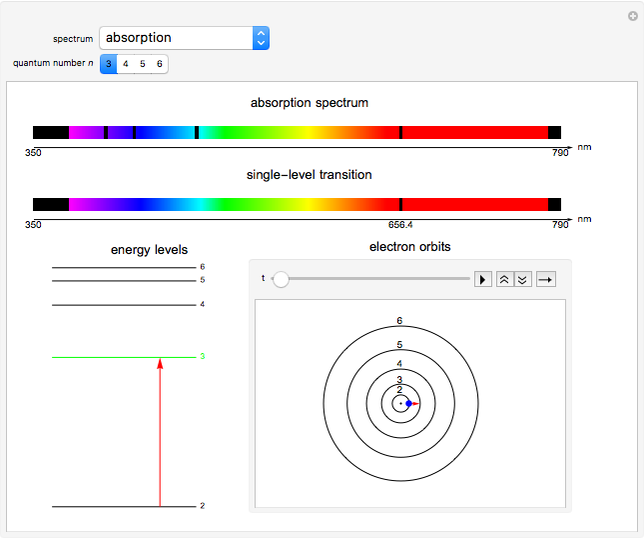
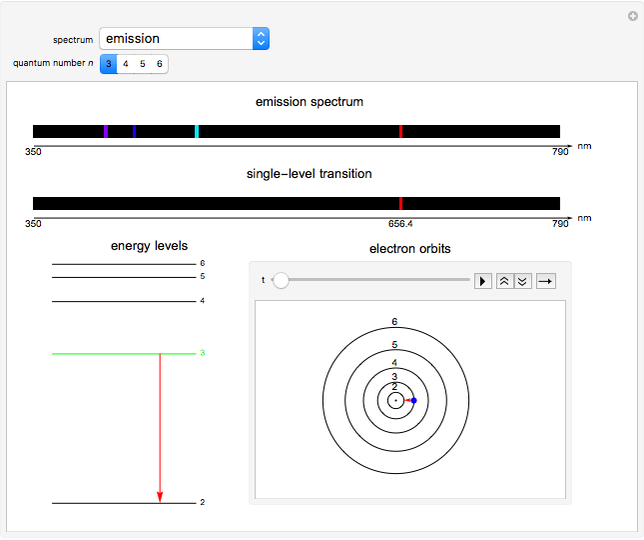
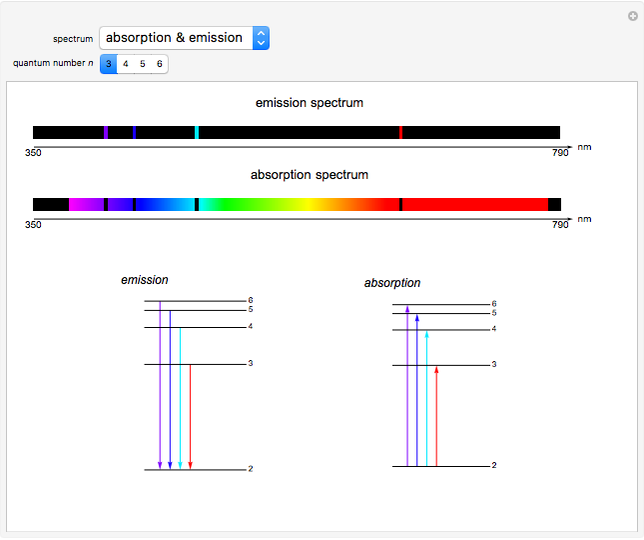
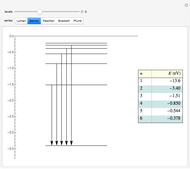
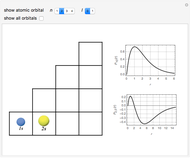
This Demonstration shows the absorption and emission lines in the visible spectrum of the hydrogen atom, according to the model proposed by Niels Bohr in 1913. This involved electronic transitions among discrete (quantized) energy levels. The energy levels are labeled by the principal quantum number  . We have analyzed the Balmer series, whose wavelengths lie in the visible region. The electron jumps corresponding to the Balmer series involve
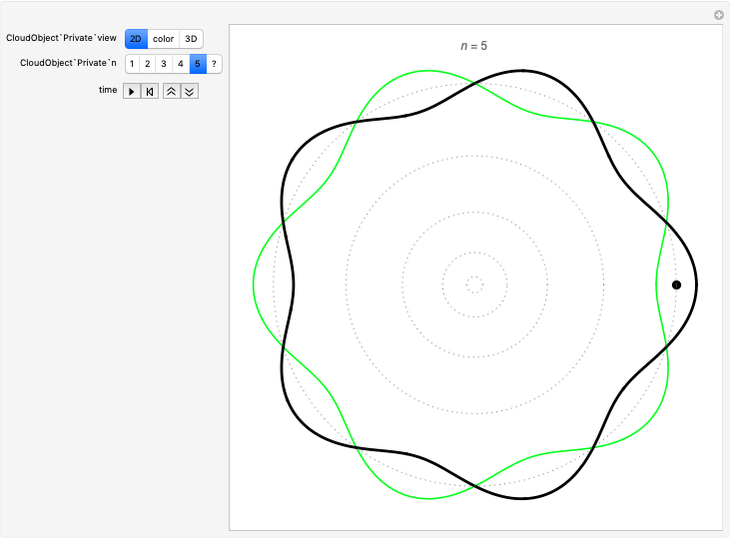
. We have analyzed the Balmer series, whose wavelengths lie in the visible region. The electron jumps corresponding to the Balmer series involve  as either the initial or the final level. These hypothetical "quantum jumps" between electron orbits can be simulated in the lower right-hand panel. The observed spectrum is represented by arrows connecting energy levels, with up arrows for absorption and down arrows for emission. You can select the quantum number
as either the initial or the final level. These hypothetical "quantum jumps" between electron orbits can be simulated in the lower right-hand panel. The observed spectrum is represented by arrows connecting energy levels, with up arrows for absorption and down arrows for emission. You can select the quantum number  . The ground state
. The ground state  is out of the energy range considered and is not seen [1].
is out of the energy range considered and is not seen [1].
Contributed by: D. Meliga and S. Z. Lavagnino (July 2016)
Additional contribution by: G. Valorio
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshot 1: light absorption involving a transition from level 2 to level 3
Snapshot 2: light emission involving a transition from level 3 to level 2
Snapshot 3: overview of the absorption and emission phenomena of the Balmer series
The visible spectrum of light from hydrogen exhibits four wavelengths: 410 nm, 434 nm, 486 nm, and 656 nm. These correspond to emissions of photons by electrons in excited states making transitions to the  state.
state.
Reference
[1] Wikipedia. "Balmer Series." (Jul 18, 2016) en.wikipedia.org/wiki/Balmer_series.
Permanent Citation