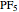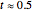Berry Pseudorotation in Phosphorus Pentafluoride

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
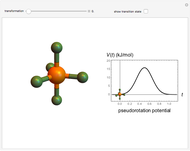
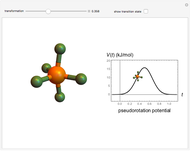
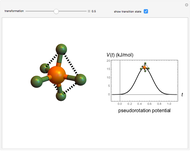
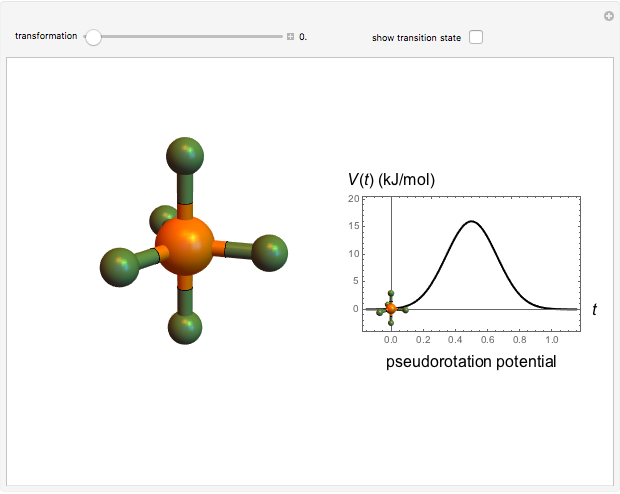
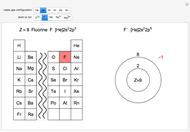
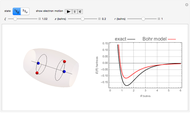
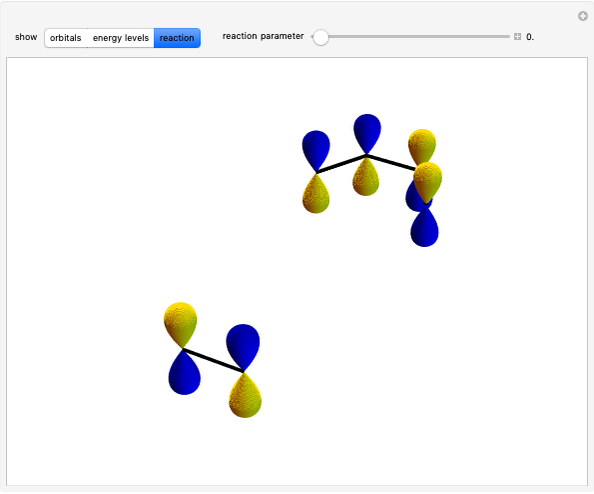
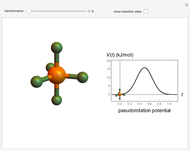
The 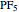 molecule has the configuration of a trigonal bipyramid, as predicted by the VSEPR model. Two of the fluorine atoms, designated as axial, are aligned with the phosphorus atom, with P-F bond distances of 1.58 Å. The remaining three fluorine atoms, designated as equatorial, are arranged in an equilateral triangle with P-F bond distances of 1.53 Å. Even though there exist two geometrically inequivalent types of fluorine atoms, the NMR spectrum of the molecule shows but a single resonance frequency. This indicates that the axial and equatorial
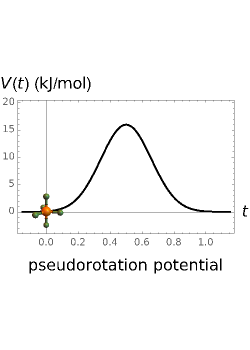
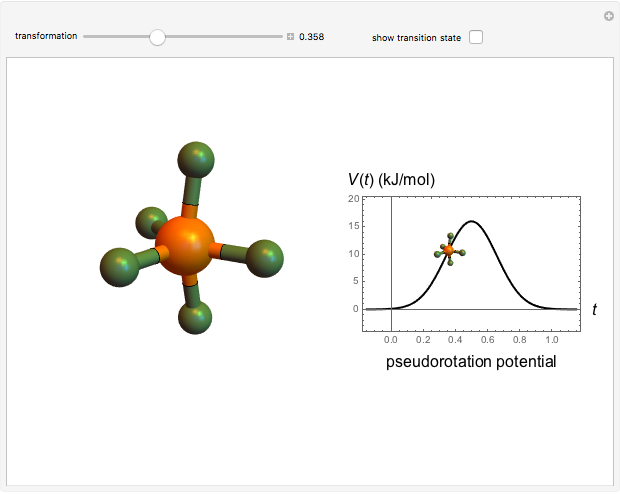
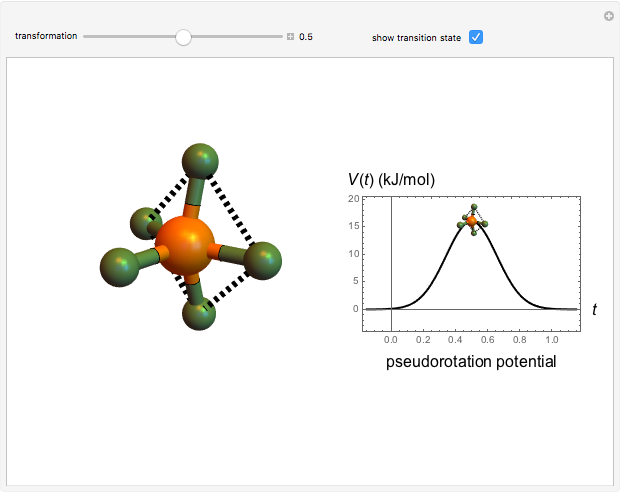
molecule has the configuration of a trigonal bipyramid, as predicted by the VSEPR model. Two of the fluorine atoms, designated as axial, are aligned with the phosphorus atom, with P-F bond distances of 1.58 Å. The remaining three fluorine atoms, designated as equatorial, are arranged in an equilateral triangle with P-F bond distances of 1.53 Å. Even though there exist two geometrically inequivalent types of fluorine atoms, the NMR spectrum of the molecule shows but a single resonance frequency. This indicates that the axial and equatorial 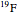 nuclei exchange their environments more rapidly than the time it takes to make the NMR measurement. Thus only a single resonance peak is seen. R. S. Berry [1] proposed a mechanism for atomic rearrangement, known as pseudorotation, since it simulates a rotation of the axial direction of the molecule. According to the proposed mechanism, one of the equatorial bonds serves as a pivot while the two axial bonds bend into two sides of an equilateral triangle. Simultaneously, the remaining two equatorial bonds line up to become the new axial bonds. Quantum-chemical computations predict a barrier of approximately 16 kJ/mol [2], which permits rapid tunneling between configurations at room temperature.
nuclei exchange their environments more rapidly than the time it takes to make the NMR measurement. Thus only a single resonance peak is seen. R. S. Berry [1] proposed a mechanism for atomic rearrangement, known as pseudorotation, since it simulates a rotation of the axial direction of the molecule. According to the proposed mechanism, one of the equatorial bonds serves as a pivot while the two axial bonds bend into two sides of an equilateral triangle. Simultaneously, the remaining two equatorial bonds line up to become the new axial bonds. Quantum-chemical computations predict a barrier of approximately 16 kJ/mol [2], which permits rapid tunneling between configurations at room temperature.
Contributed by: S. M. Blinder (August 2018)
Open content licensed under CC BY-NC-SA
Details
References
[1] R. S. Berry, "Correlation of Rates of Intramolecular Tunneling Processes, with Application to Some Group V Compounds," The Journal of Chemical Physics, 32(3), 1960 pp. 933–938. doi:10.1063/1.1730820.
[2] C. J. Marsden, "Pseudorotation Pathway and Quadratic Force Field for 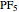 , by ab initio Calculations," Journal of the Chemical Society, Chemical Communications, 1984(7), pp. 401–402. doi:10.1039/C39840000401.
, by ab initio Calculations," Journal of the Chemical Society, Chemical Communications, 1984(7), pp. 401–402. doi:10.1039/C39840000401.
Snapshots
Permanent Citation