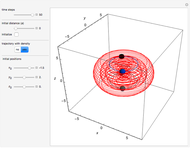
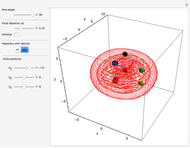
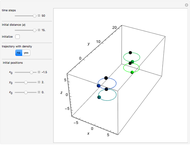
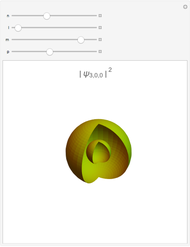
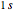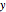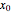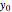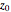Bohm Trajectories in an LCAO Approximation for the Hydrogen Molecule 

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
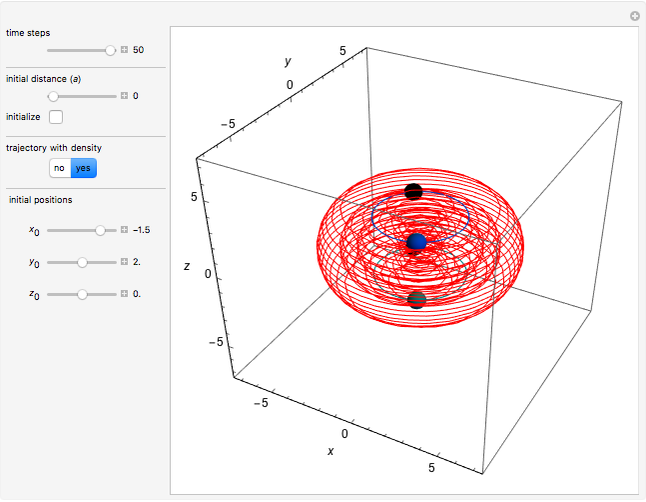
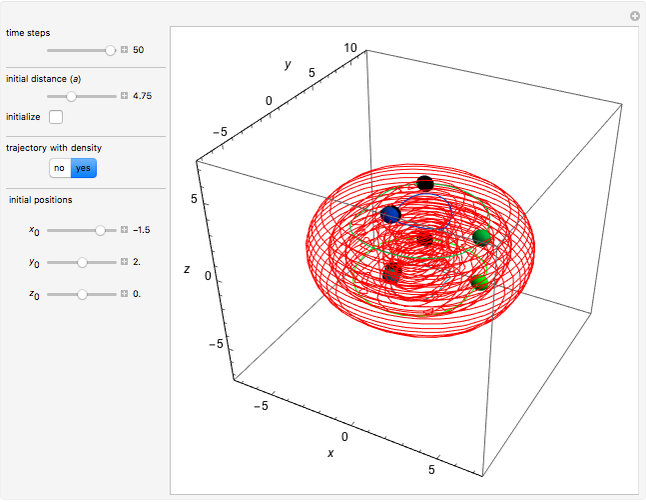
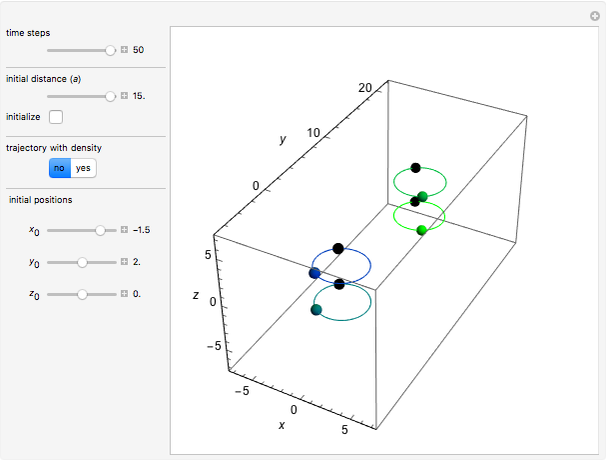
In quantum chemistry, linear combinations of atomic orbitals (LCAO) have long been used to construct molecular orbitals. This Demonstration considers the Bohm trajectories of two initially separated excited metastable hydrogen atoms, separated by an internuclear distance  . Keep in mind that in this special case, the standard LCAO method must be adjusted to fit, and it does not give exact solutions to the Schrödinger equation, but only first approximations.
. Keep in mind that in this special case, the standard LCAO method must be adjusted to fit, and it does not give exact solutions to the Schrödinger equation, but only first approximations.
Contributed by: Klaus von Bloh (December 2017)
Open content licensed under CC BY-NC-SA
Snapshots
Details
With the internuclear distance parameter 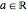 , since the Coulomb potential
, since the Coulomb potential
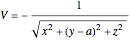
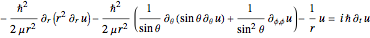 .
.
For simplicity, set the reduced mass  and
and  equal to 1 (atomic units). This leads to the time-dependent wavefunction with the associated Laguerre polynomials
equal to 1 (atomic units). This leads to the time-dependent wavefunction with the associated Laguerre polynomials 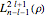 and the spherical harmonics
and the spherical harmonics 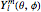 :
:
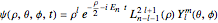 ,
,
with the energy eigenvalue 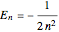 and
and 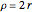 . The principal quantum number
. The principal quantum number  determines the energy,
determines the energy, 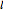 is called the orbital angular momentum quantum number and
is called the orbital angular momentum quantum number and 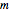 is the magnetic quantum number. To get Bohm trajectories, the hydrogen atom has to be excited because the magnetic number
is the magnetic quantum number. To get Bohm trajectories, the hydrogen atom has to be excited because the magnetic number 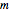 must be
must be 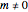 , which is only fulfilled for states with
, which is only fulfilled for states with 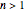 . When
. When 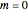 , the electron is at rest; for a stationary state with
, the electron is at rest; for a stationary state with 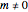 , the electron orbits the
, the electron orbits the  axis along a circle of constant radius and with constant angular speed, depending on the sign and magnitude of
axis along a circle of constant radius and with constant angular speed, depending on the sign and magnitude of 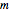 [2].
[2].
For  (
(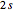 state), the energy equals
state), the energy equals 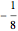 . For
. For  ,
,  ,
, 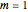 , in Cartesian coordinates with
, in Cartesian coordinates with
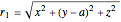 ,
,
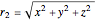 ,
,
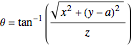 and
and
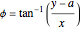 ,
,
the spatial wavefunction on each of the two hydrogen atoms forms a linear combination that leads to LCAO-MO-like application to 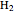 , which is not the standard LCAO-MO method, with:
, which is not the standard LCAO-MO method, with:
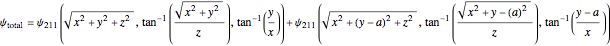 .
.
For this very special case, the wavefunction becomes:
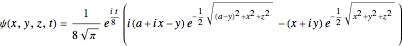 .
.
For  , the wavefunction becomes an exact solution of the Schrödinger equation. From the wavefunction for
, the wavefunction becomes an exact solution of the Schrödinger equation. From the wavefunction for  , the equation for the phase function
, the equation for the phase function 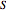 follows:
follows:
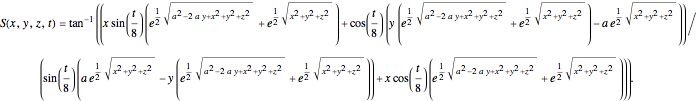
In Cartesian coordinates, the 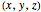 components of the velocity could be declared by the gradient of the total phase function from the total wave function in the eikonal form, which lead in this special case to a corresponding autonomous differential equation system:
components of the velocity could be declared by the gradient of the total phase function from the total wave function in the eikonal form, which lead in this special case to a corresponding autonomous differential equation system:
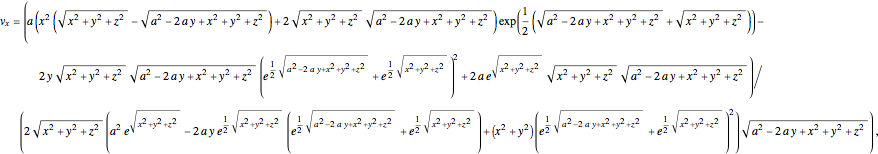
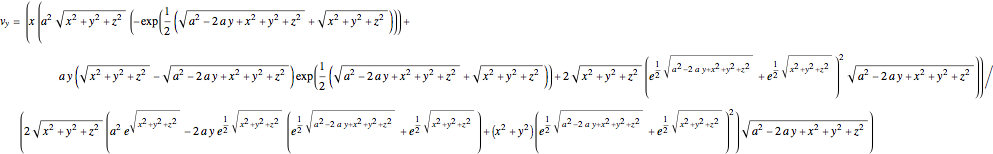
and
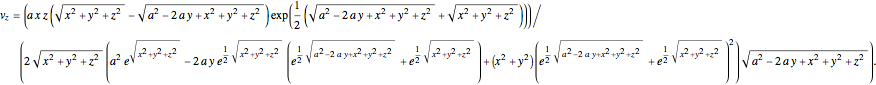
For the limiting case 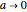 and for the asymptotic behavior
and for the asymptotic behavior 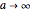 , the
, the 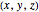 components of the velocity become:
components of the velocity become:
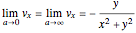 ,
,
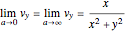 and
and
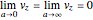 .
.
For more detailed information about the hydrogen-like atom and Bohm theory, see [1–3, 6, 7], and for a general introduction to Bohmian mechanics, see [2, 4, 5].
In the program, to make the results more accurate, increase PlotPoints, MaxRecursion, AccuracyGoal, PrecisionGoal and MaxSteps.
References
[1] C. Dewdney and Z. Malik, "Angular-Momentum Measurement and Nonlocality in Bohm’s Interpretation of Quantum Theory," Physical Review A, 48(5), 1993 pp. 3513–3524. doi:10.1103/PhysRevA.48.3513.
[2] P. Holland, The Quantum Theory of Motion: An Account of the de Broglie–Bohm Causal Interpretation of Quantum Mechanics, Cambridge: Cambridge University Press, 1993.
[3] C. Colijn and E. R. Vrscay, "Spin-Dependent Bohm Trajectories for Hydrogen Eigenstates," Physics Letters A, 300(4–5), 2002 pp. 334–340. doi:10.1016/S0375-9601(02)00824-1.
[4] "Bohmian-Mechanics.net." (Dec 20, 2017) www.bohmian-mechanics.net/index.html.
[5] S. Goldstein. "Bohmian Mechanics." The Stanford Encyclopedia of Philosophy. (Dec 20, 2017)plato.stanford.edu/entries/qm-bohm.
[6] L. P. Yatsenko, V. I. Romanenko, B. W. Shore, T. Halfmann and K. Bergmann,"Two-Photon Excitation of the Metastable 2s State of Hydrogen Assisted by Laser-Induced Chirped Stark Shifts and Continuum Structure," Physical Review A, 71(3), 2005 pp. 1–10. doi:10.1103/PhysRevA.71.033418.
[7] Wikipedia. "Hydrogen Atom." (Dec 20, 2017) en.wikipedia.org/wiki/Hydrogen_atom.
Permanent Citation