Construct an x-y Diagram for Flash Distillation

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
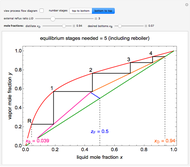
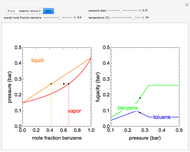
This Demonstration leads you through a step-by-step procedure to generate an 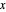 -
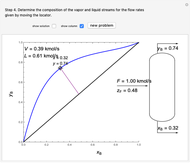
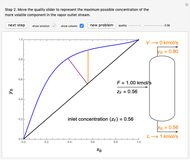
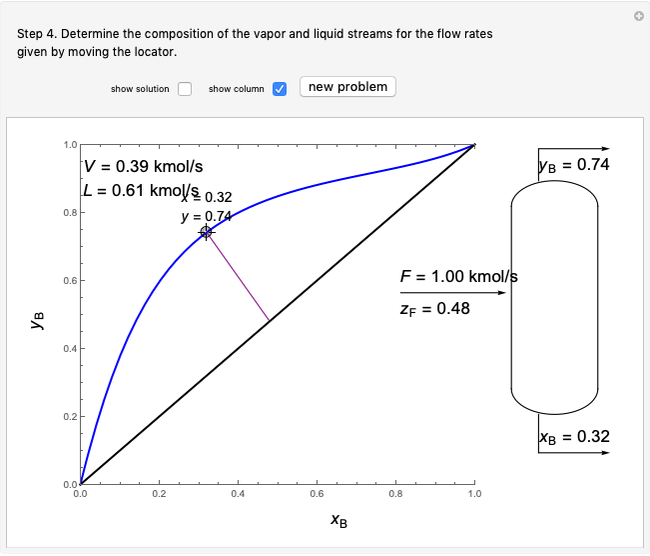
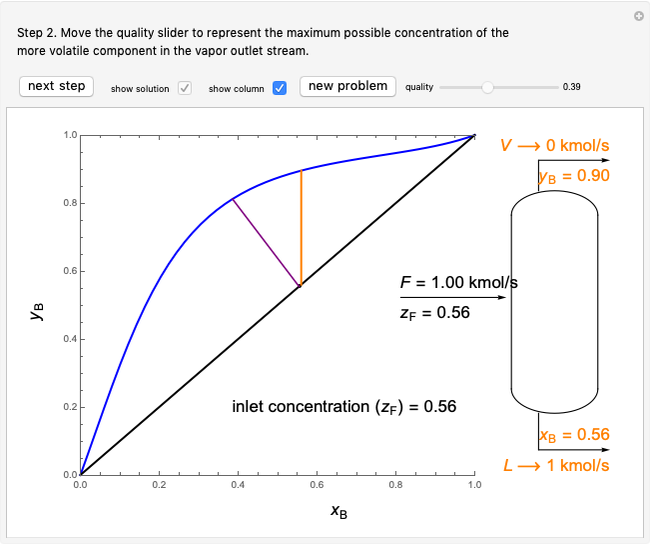
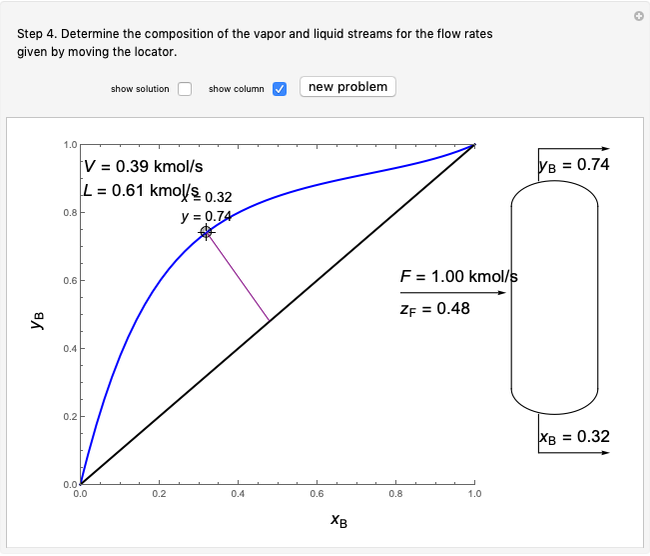
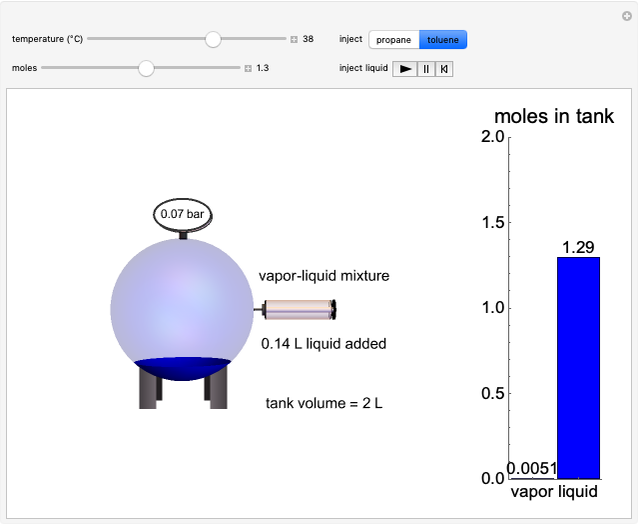
-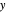 graph for a binary vapor-liquid equilibrium and then determines outlet compositions for a flash distillation column. Points are moved using a locator on the graph or moving the slider to select the desired quality (defined here as vapor flow rate divided by feed flow rate). After each solution is ready, select "show solution" to see the correct solution and then select "next step". Selecting "show column" presents a representation of the column with flow rates and mole fractions (when "show solution" is selected). At any time, "new problem" can be clicked to generate a new set of values.
graph for a binary vapor-liquid equilibrium and then determines outlet compositions for a flash distillation column. Points are moved using a locator on the graph or moving the slider to select the desired quality (defined here as vapor flow rate divided by feed flow rate). After each solution is ready, select "show solution" to see the correct solution and then select "next step". Selecting "show column" presents a representation of the column with flow rates and mole fractions (when "show solution" is selected). At any time, "new problem" can be clicked to generate a new set of values.
Contributed by: Neil Hendren (December 2020)
Additional contributions by: John L. Falconer
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
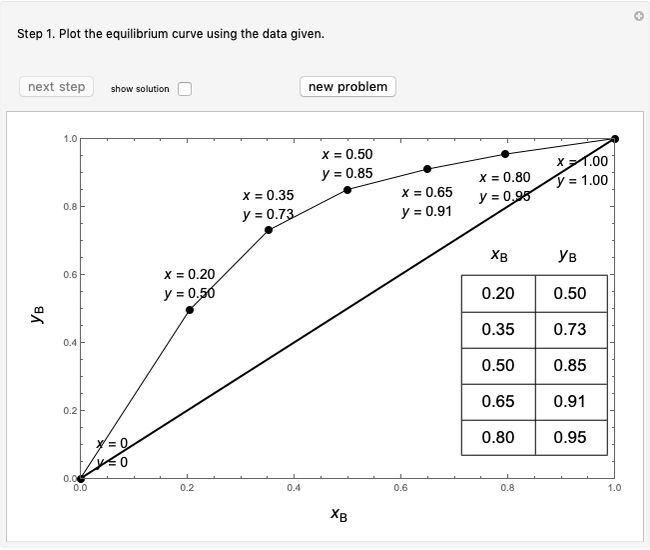
Flash distillation (also known as flash evaporation) is steady-state, single-stage distillation. In this Demonstration, the liquid feed contains two components. An adiabatic throttle reduces the pressure and a fraction of the liquid feed evaporates to maintain constant enthalpy at the lower pressure. Phase equilibrium data at the outlet pressure helps determine the composition and flow rates of the outlet streams.
The 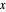 -
-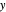 equilibrium curve is generated using the modified Raoult's law:
equilibrium curve is generated using the modified Raoult's law:
(1) 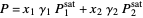 ,
,
(2) 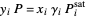 ,
,
where 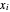 and
and 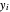 are the liquid and vapor mole fractions (
are the liquid and vapor mole fractions (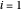 for the more volatile component),
for the more volatile component), 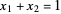 ,
, 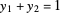 ,
, 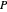 is total pressure and
is total pressure and 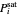 is the saturation pressure, which is calculated using the Antoine equation:
is the saturation pressure, which is calculated using the Antoine equation:
(3) 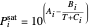 .
.
The activity coefficients 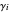 in this simulation are pseudorandom.
in this simulation are pseudorandom.
Once the equilibrium curve has been determined, a system can be fully defined by mass balances:
(6) 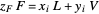 ,
,
(7) 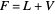 ,
,
where 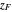 ,
, 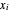 and
and 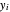 are the mole fractions of the more volatile component in the feed, bottoms (liquid) and distillate (vapor) streams, respectively. The flow rates (
are the mole fractions of the more volatile component in the feed, bottoms (liquid) and distillate (vapor) streams, respectively. The flow rates (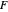 ,
, 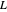 ,
, 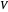 ) are for the feed, bottoms and distillate, respectively.
) are for the feed, bottoms and distillate, respectively.
In the case of this Demonstration, 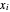 and
and 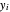 are determined graphically. This is done by reading the
are determined graphically. This is done by reading the 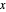 value and
value and 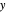 value at the intersection of the
value at the intersection of the  -line with the equilibrium curve. The
-line with the equilibrium curve. The  -line equation is a function of the vapor and liquid flow rates and feed conditions:
-line equation is a function of the vapor and liquid flow rates and feed conditions:
(8) 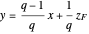 ,
,
(9) 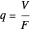 .
.
Permanent Citation