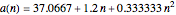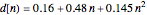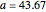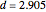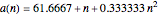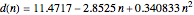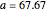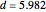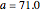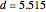Curve Fitting to Find Missing Elements in Mendeleev's Periodic Table

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
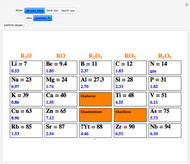
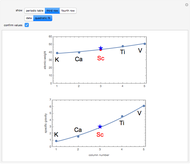
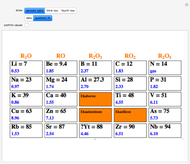
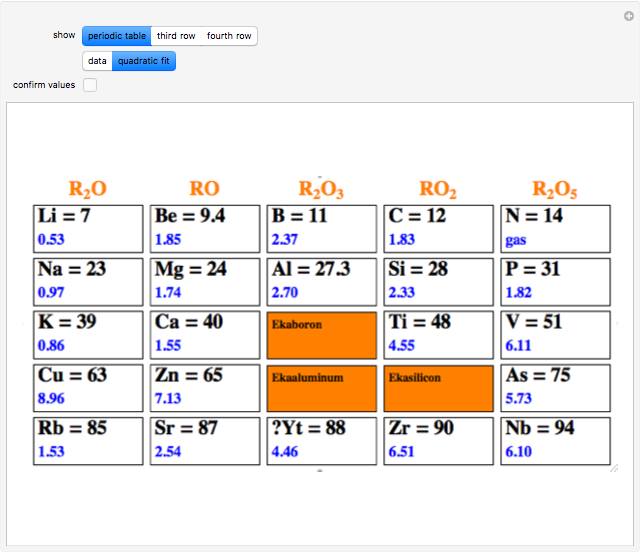
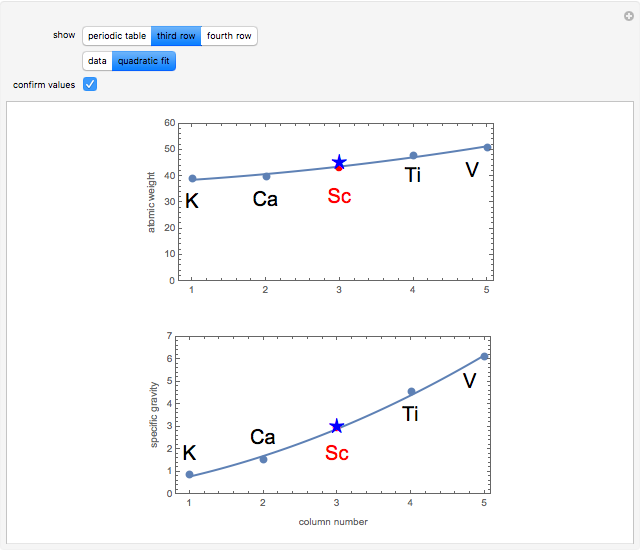
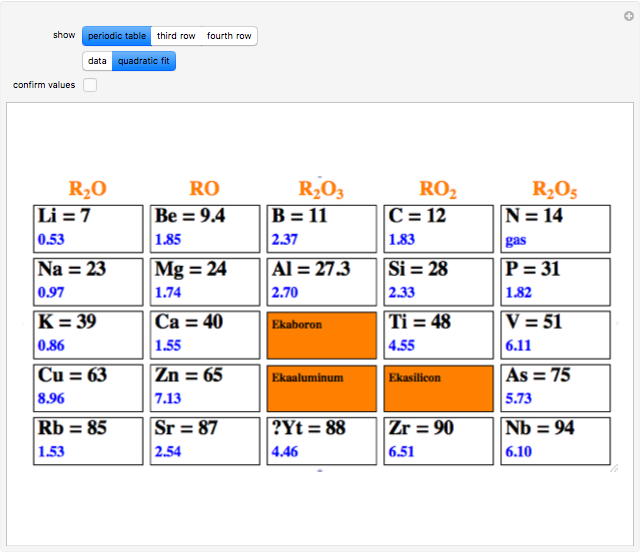
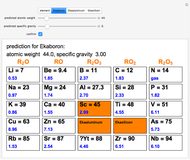
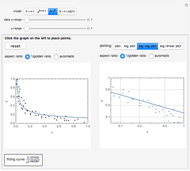
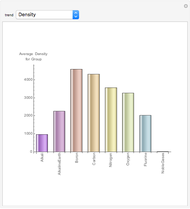
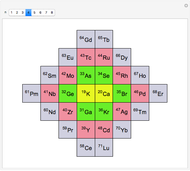
The first graphic (click "periodic table") shows a 5×5 fragment of Mendeleev's periodic table of the elements, ordered by increasing atomic weights, such that elements with chemical similarities occur in vertical groups. The headings give the formulas for the most stable oxide for elements in each group. Also shown in blue are the specific gravities of the elements. Mendeleev surmised that there must be missing elements in the three orange boxes, to which he gave the provisional names Ekaboron, Ekaaluminum, and Ekasilicon.
[more]
Contributed by: Charles Keller (Coastal Carolina University) and S. M. Blinder (November 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference
[1] S. M. Blinder, Introduction to Quantum Mechanics, Amsterdam: Elsevier, 2004 p. 124.