Diffusion in Solids

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
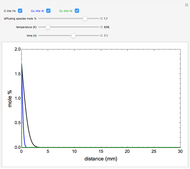
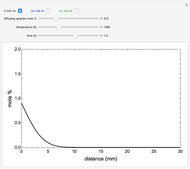
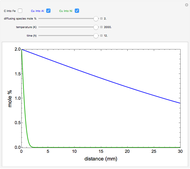
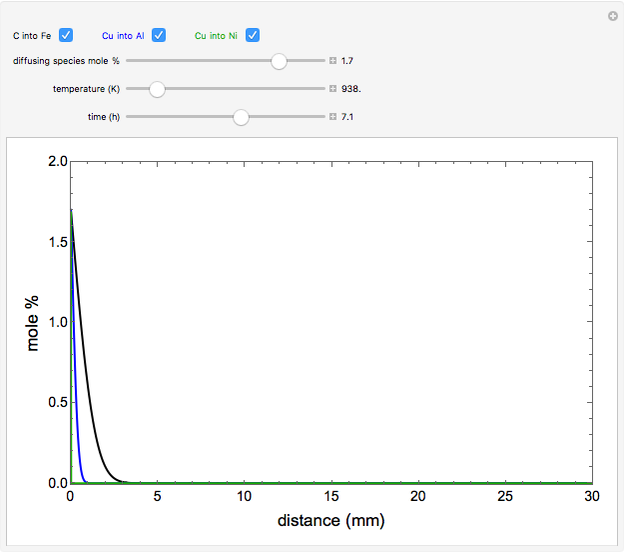
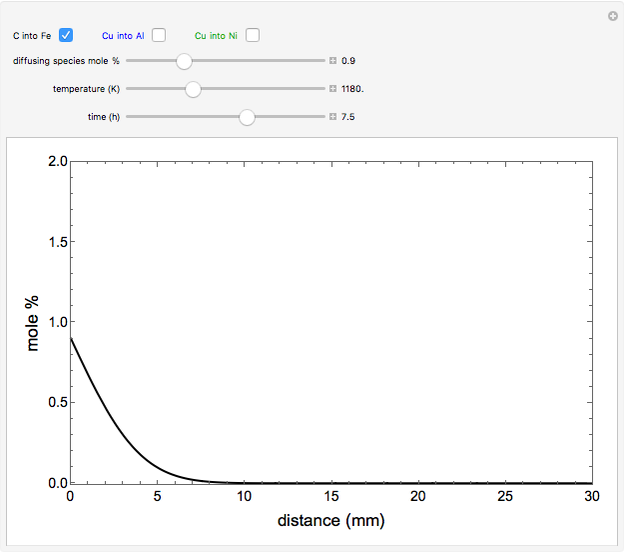
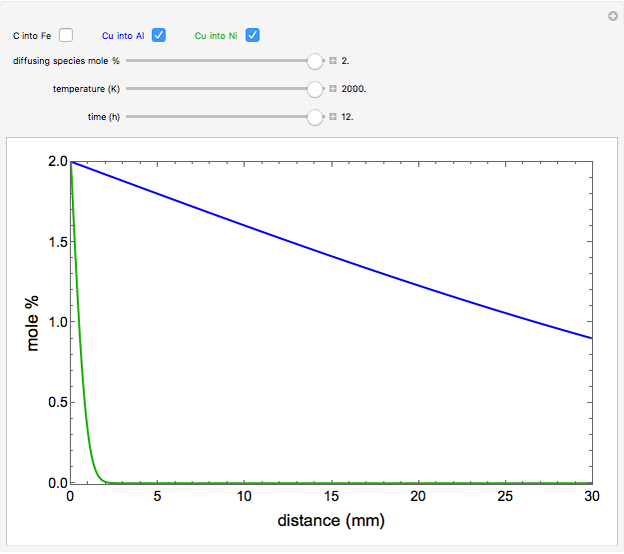
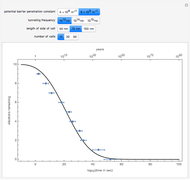
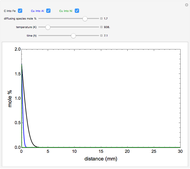
The mole percentage of a solute in a semi-infinite solid block is calculated as a function of distance from the surface. Carbon and copper are the diffusing species and their surface concentrations are fixed using a slider. Display the diffusing systems by checking the boxes for carbon into iron (C into Fe), copper into aluminum (Cu into Al) and copper into nickel (Cu into Ni). Use sliders to vary the temperature of the block and the diffusion time.
Contributed by: Nathan S. Nelson (July 2015)
Additional contributions by: Rachael L. Baumann and John L. Falconer
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
The concentration 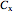 is:
is:
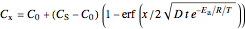 ,
,
where 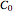 is the initial concentration of the diffusing molecule in the bulk solid,
is the initial concentration of the diffusing molecule in the bulk solid, 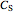 is the external surface concentration of the diffusing molecule,
is the external surface concentration of the diffusing molecule,  is the distance into the bulk solid,
is the distance into the bulk solid, 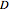 is the diffusion coefficient characteristic of the diffusing molecule and the solid,
is the diffusion coefficient characteristic of the diffusing molecule and the solid, 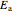 is activation energy for diffusion in the specific system,
is activation energy for diffusion in the specific system, 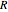 is the gas constant,
is the gas constant, 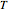 is temperature and
is temperature and  is time.
is time.
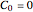 for this Demonstration. That is, at time zero, the concentration of the diffusing species is zero everywhere except at the surface.
for this Demonstration. That is, at time zero, the concentration of the diffusing species is zero everywhere except at the surface.
The following parameters are used:
carbon diffusing into iron (C into Fe):
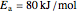 ,
,
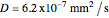
copper diffusing into aluminum (Cu into Al):
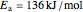 ,
,
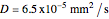
copper diffusing into nickel (Cu into Ni):
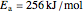 ,
,
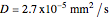 .
.
Permanent Citation