Extraction of Acetic Acid from Water Using Isopropyl Ether

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
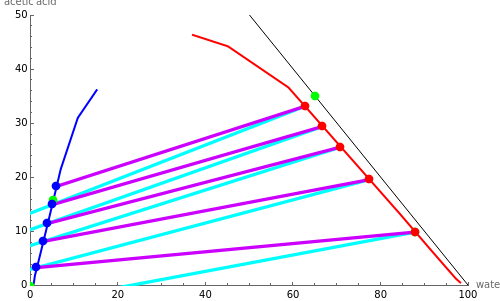
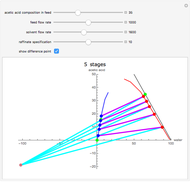
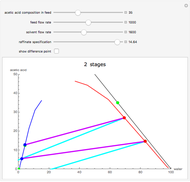
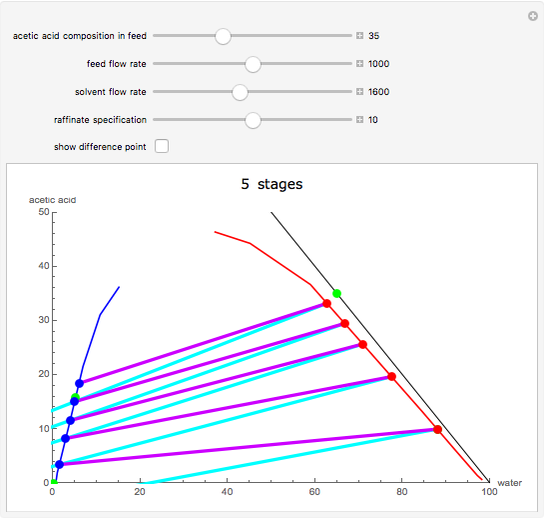
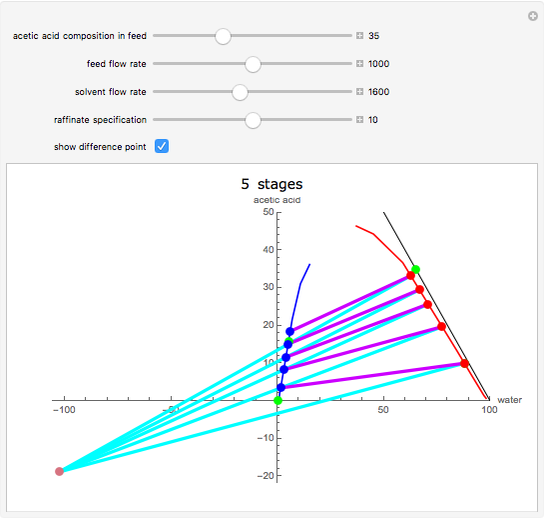
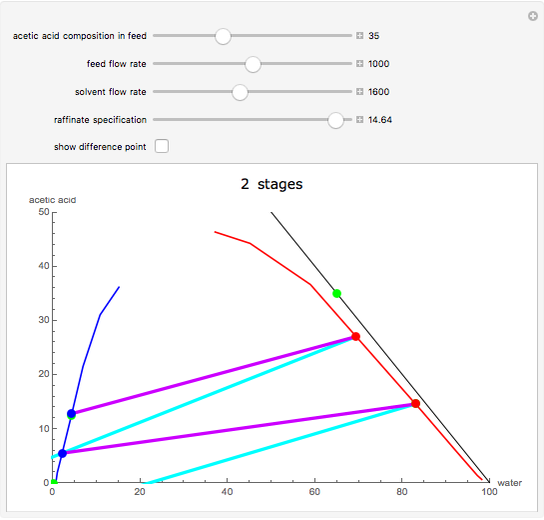
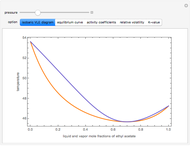
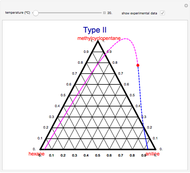
This Demonstration is a study of the separation of acetic acid from water using isopropyl ether for a single-feed countercurrent extractor. You can vary the flow rate and acetic acid composition of the feed, the flow rate of the solvent, which is pure isopropyl ether, and the raffinate composition in acid. The cyan and purple lines are the operating and tie lines, respectively. The red and blue dots are on the raffinate and extract curves. They correspond to compositions of the raffinate and extract streams at various locations in the extractor. Stages are stepped off using the tie lines and the operating lines alternatively until the raffinate composition is lower than the specification. The number of equilibrium stages is displayed.
Contributed by: Housam Binous (September 2007)
Open content licensed under CC BY-NC-SA
Snapshots
Details
The graphical method derived by Hunter and Nash (1) and later by Kinney (2) is used to obtain the number of equilibrium stages required to achieve a particular raffinate specification. This separation is usually difficult to realize with distillation due to the presence of a severe tangent pinch at high compositions of water, which prevents the distillate from being acid-free. A graphical solution performed by hand calculation has been presented by Wankat (3). This problem is solved more easily and accurately using the graphical approach of Hunter and Nash. Equilibrium data at 1 atm and 25°C is given by Wankat and can be used to plot the tie lines.
References
[1] T. G. Hunter and A. W. Nash, "The Application of Physico-Chemical Principles to the Design of Liquid-Liquid Contact Equipment, Part II: Application of Phase-Rule Graphical Method," J. Soc. Chem. Ind., 53, 1934 pp. 95T–102T.
[2] G. F. Kinney, "Leaching Calculations: A Note on the Graphical Method," Ind. Eng. Chem., 34, 1942 pp. 1102–1104.
[3] P. C. Wankat, Equilibrium Staged Separations, New Jersey: Prentice Hall, 1988 (example 18–2, pp. 595, and example 18–3, pp. 609).
[4] H. Binous, "Equilibrium Staged Separations using Matlab® and Mathematica®," Chemical Engineering Education, 42(2), 2008 pp. 69-73.
[5] H. Binous, "Liquid-Liquid Equilibrium and Extraction using Mathematica®," Computers in Education Journal, 16(1), 2006 pp. 78-81.
Permanent Citation
"Extraction of Acetic Acid from Water Using Isopropyl Ether"
http://demonstrations.wolfram.com/ExtractionOfAceticAcidFromWaterUsingIsopropylEther/
Wolfram Demonstrations Project
Published: September 28 2007