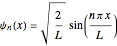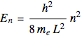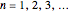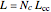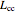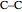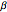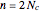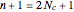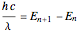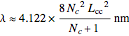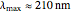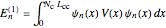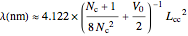Free-Electron Model for Linear Polyenes

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
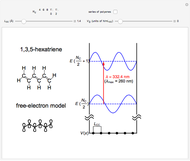
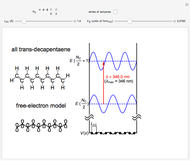
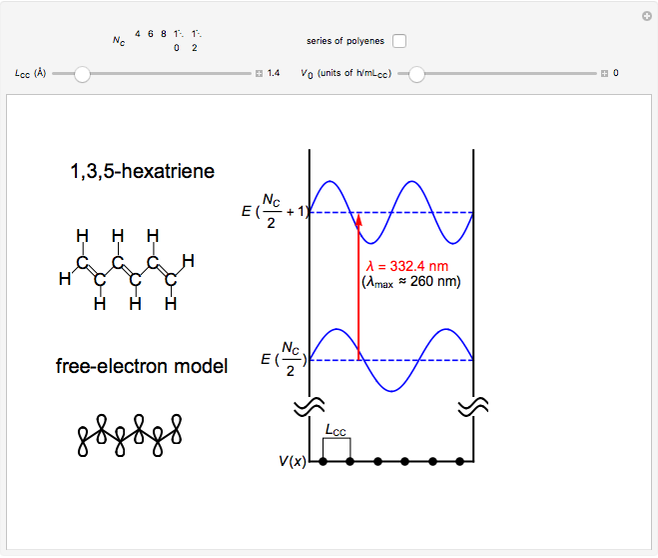
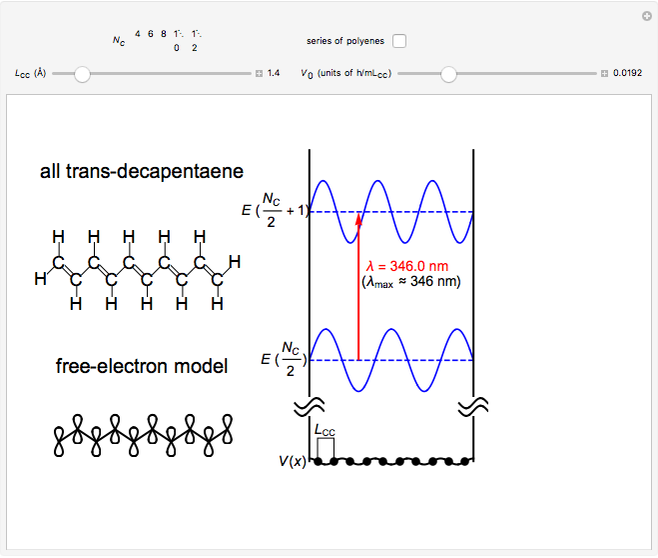
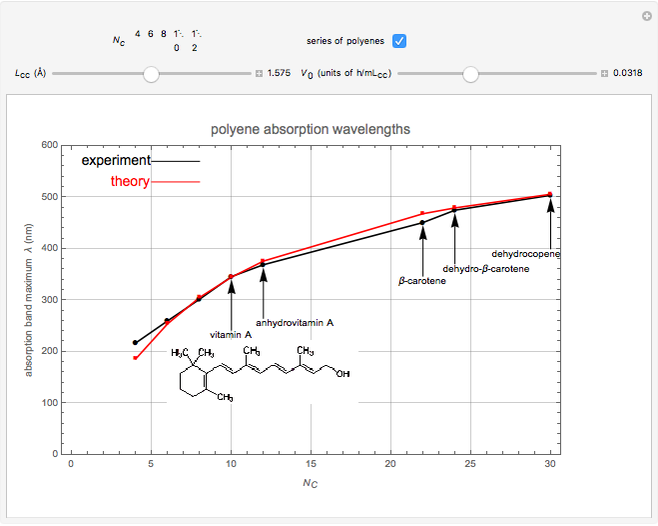
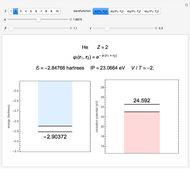
The simplest nontrivial application of the Schrödinger equation is the one-dimensional particle in a box. Remarkably, this simple system can provide a useful model for a significant chemical problem—the structure of linear polyene molecules. A polyene is a hydrocarbon with alternating single and double carbon-carbon bonds, the simplest example being butadiene 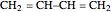 . The series continues with hexatriene, octatetrene, and so on, with the generic structure
. The series continues with hexatriene, octatetrene, and so on, with the generic structure 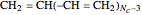 , a chain of
, a chain of 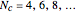 conjugated carbon atoms. After all the single bonds are accounted for, each carbon atom contributes one
conjugated carbon atoms. After all the single bonds are accounted for, each carbon atom contributes one 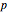 -electron. Linear combinations of these
-electron. Linear combinations of these 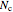 atomic
atomic 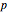 -orbitals can form
-orbitals can form 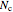
 -molecular orbitals (MOs), delocalized over the entire length of the molecule.
-molecular orbitals (MOs), delocalized over the entire length of the molecule.
Contributed by: S. M. Blinder (April 2014)
Open content licensed under CC BY-NC-SA
Snapshots
Details
References
[1] S. M. Blinder, Introduction to Quantum Mechanics: In Chemistry, Materials Science, and Biology, Amsterdam: Elsevier Academic Press, 2004, pp. 37–39.
[2] S. Huzinaga and T. Hasino, "Electronic Energy Levels of Polyene Chains," Progress of Theoretical Physics, 18(6), 1957 pp. 649–658. doi:10.1143/PTP.18.649.
Permanent Citation