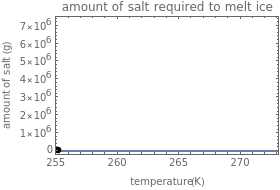
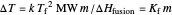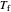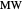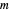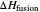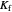Freezing Point Depression: How Much Salt Will Melt Ice?
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
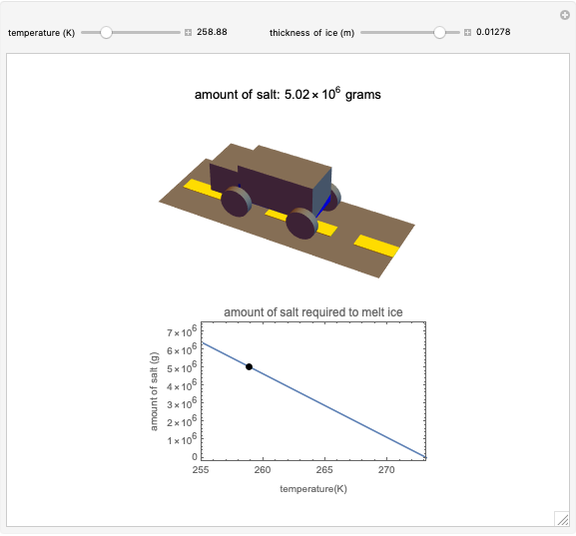
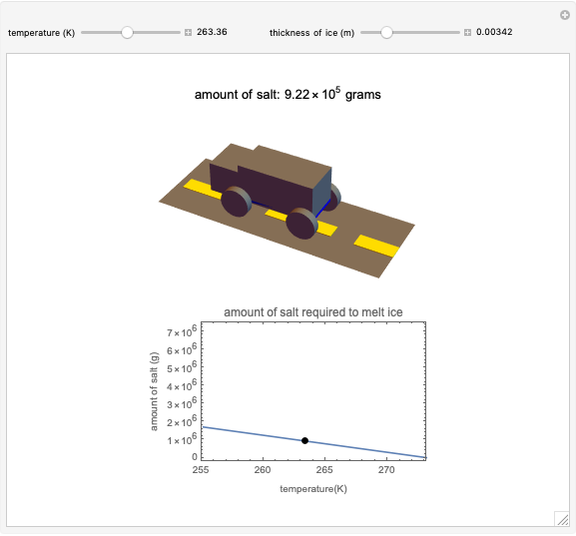
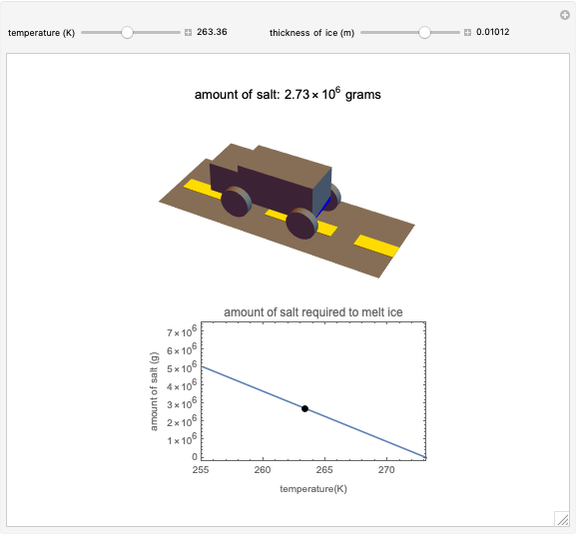
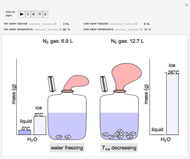
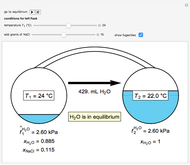
Use the sliders to set the temperature and the thickness of ice on a road. The equation for freezing point depression is given by
[more]
Contributed by: Jessica Baker, Elena Romund and Taylor Coates (April 2019)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference
[1] R. Chang, Physical Chemistry for the Biosciences, Sansalito, CA: University Science Books, 2005 pp. 138–147, 507.
Permanent Citation