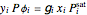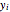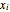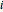High Pressure Vapor-Liquid Equilibrium Data of a Binary Mixture of Chloroform and Acetone

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
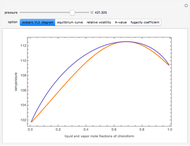
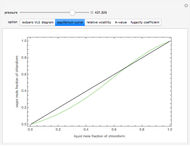
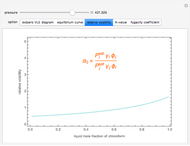
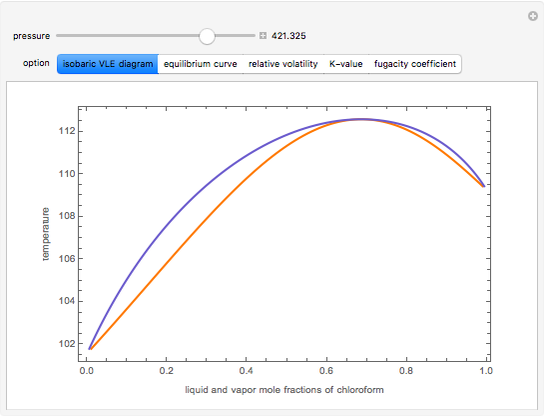
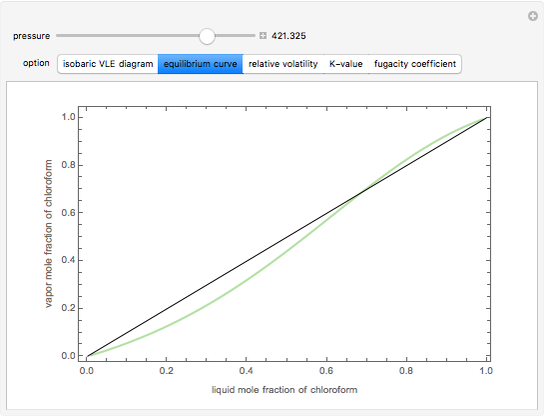
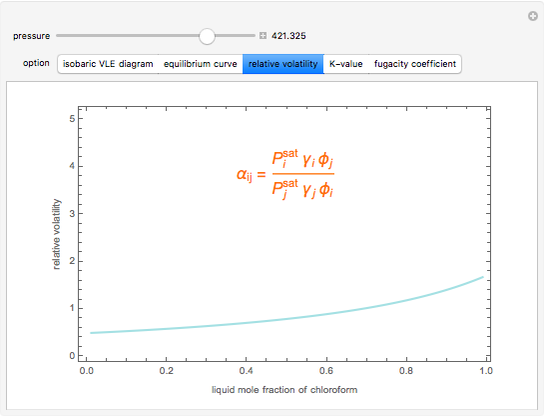
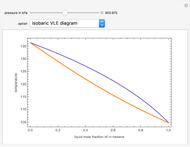
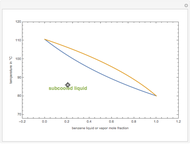
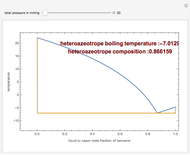
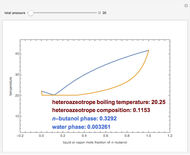
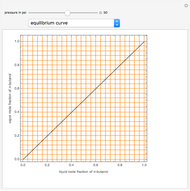
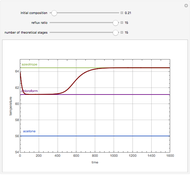
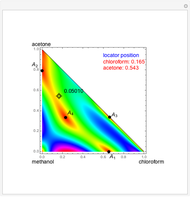
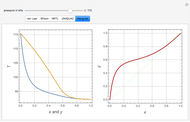
Consider a binary mixture composed of chloroform and acetone. This mixture exhibits a negative azeotrope. This Demonstration computes and plots the isobaric vapor-liquid equilibrium (VLE) diagram, the equilibrium curve, the relative volatility, the 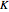 -value, and the gas phase fugacity coefficients for user-specified values of the pressure. This Demonstration takes into account the deviation from ideal behavior in the gas phase by including gas phase fugacity coefficients. Thus high-pressure VLE data can be obtained. This Demonstration does not allow for the Poynting Factor Correction (or POY).
-value, and the gas phase fugacity coefficients for user-specified values of the pressure. This Demonstration takes into account the deviation from ideal behavior in the gas phase by including gas phase fugacity coefficients. Thus high-pressure VLE data can be obtained. This Demonstration does not allow for the Poynting Factor Correction (or POY).
Contributed by: Housam Binous (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Permanent Citation