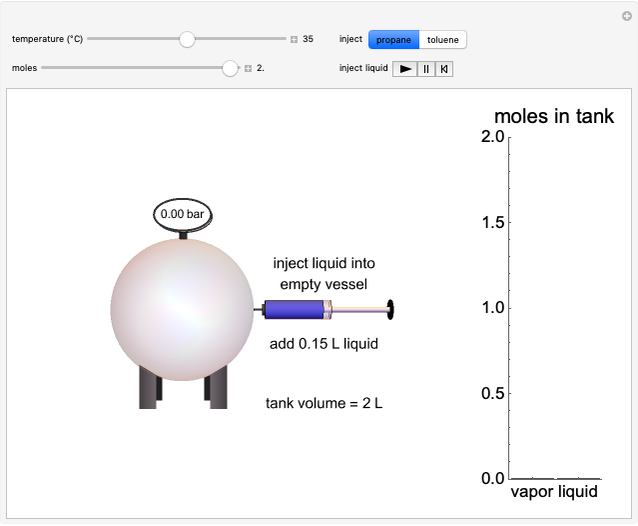
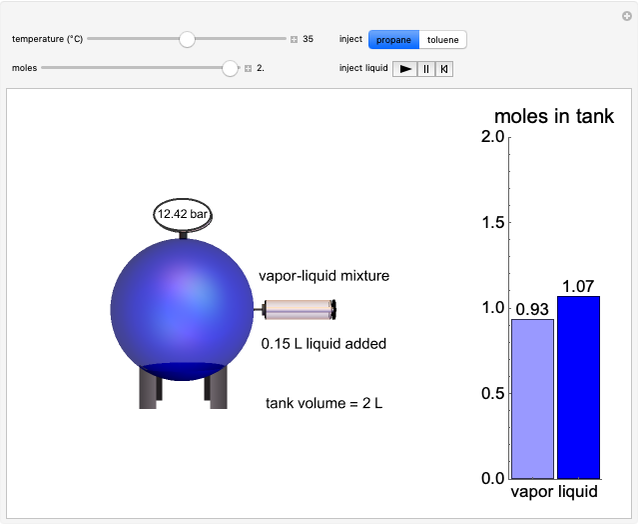
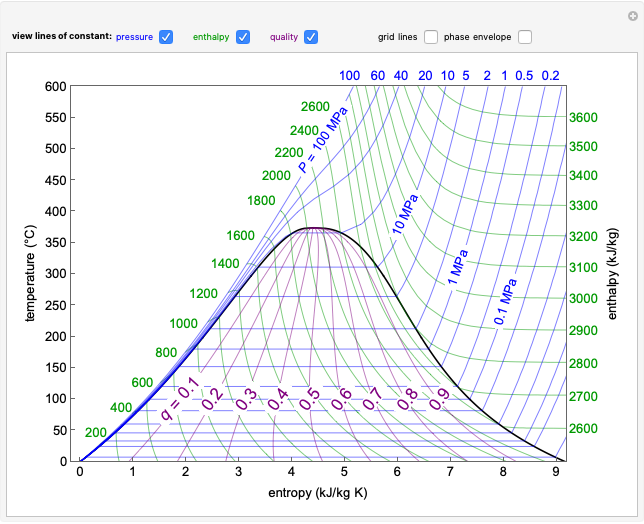
Injecting a Liquid into an Evacuated Tank

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
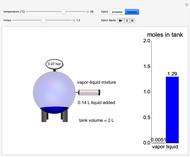
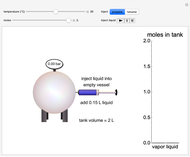
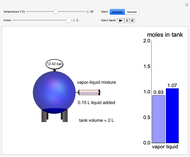
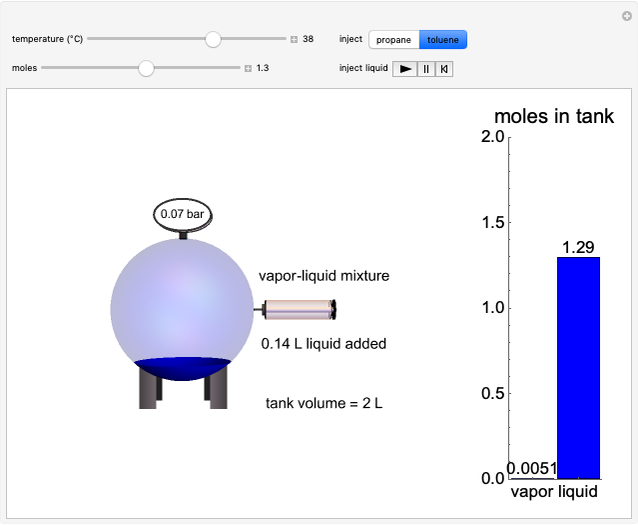
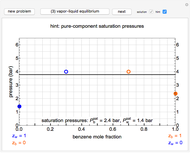
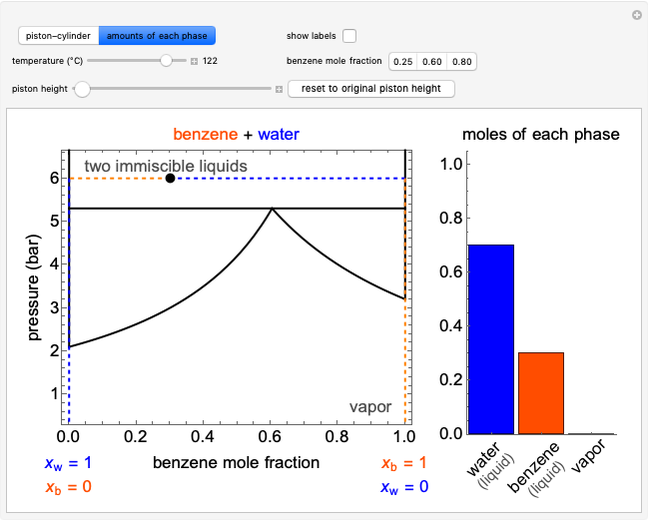
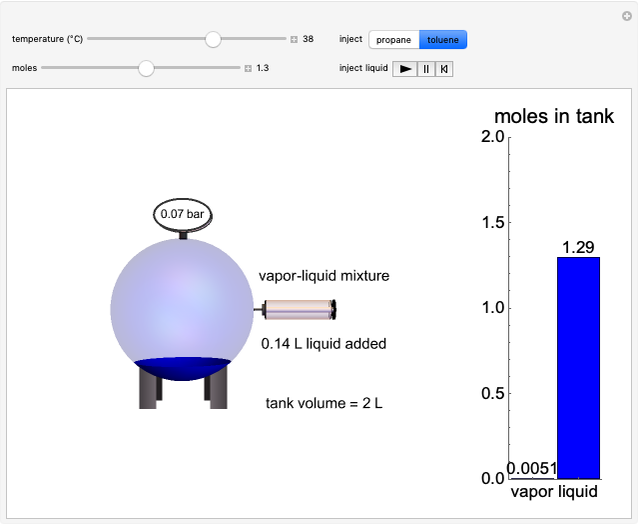
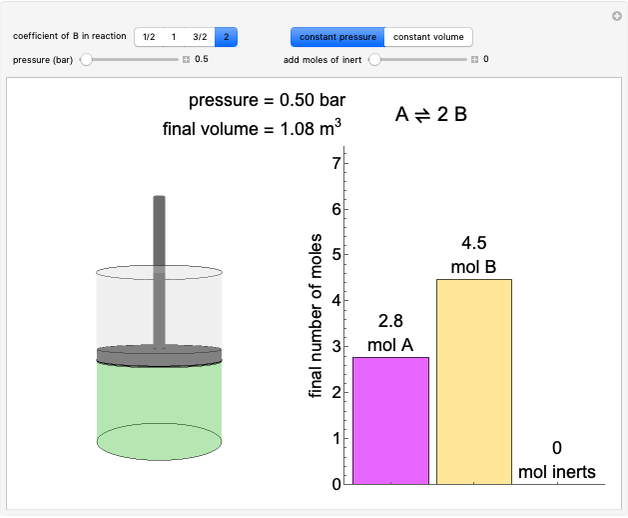
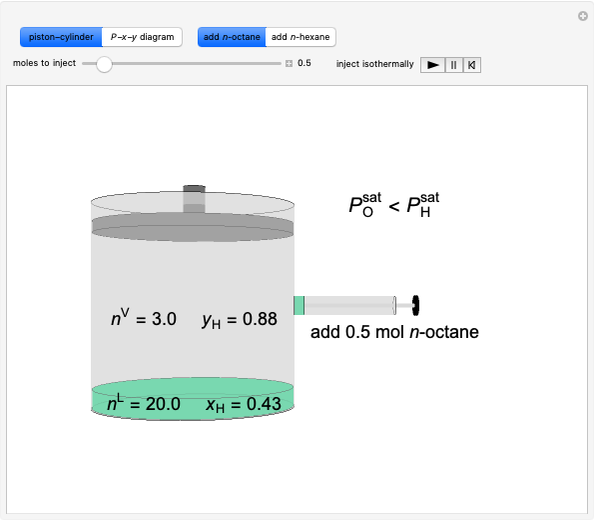
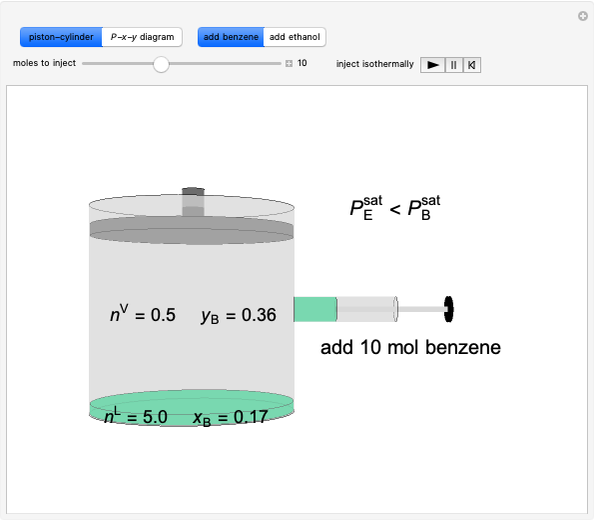
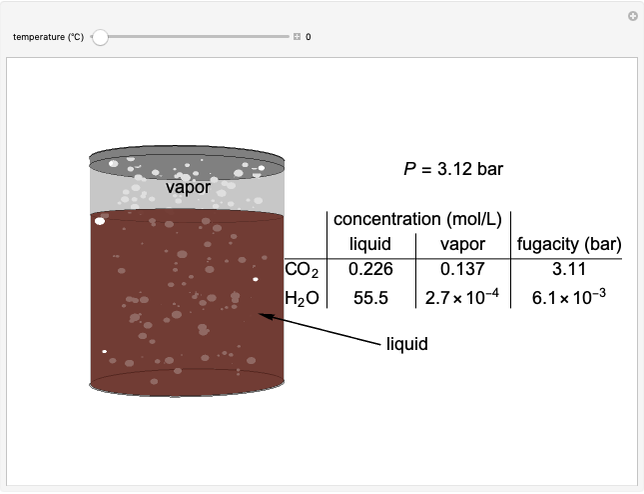
In this Demonstration, liquid propane or liquid toluene is injected into an evacuated 2-L spherical tank that is held at constant temperature. When propane is injected, it either vaporizes completely or forms two phases in vapor-liquid equilibrium (VLE), depending on the number of moles injected and the temperature (control both with sliders). Because toluene has a much lower saturation pressure, only a small amount of injected toluene liquid vaporizes. When the component is in VLE, the tank pressure equals the saturation pressure; otherwise, the pressure is calculated using the ideal gas law. The pressure is displayed at the top of the tank. Click the play button next to "inject liquid" to inject liquid into the tank. The intensity of the blue color of the vapor is proportional to the vapor density. The liquid volume in the tank is exaggerated relative to the vapor volume for better visualization. The bar graph on the right shows the number of moles of liquid and vapor in the tank.
Contributed by: Rachael L. Baumann (June 2015)
Additional contributions by: John L. Falconer, Derek M. Machalek, Nathan S. Nelson, Neil Hendren and Garrison J. Vigil
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
When propane or toluene is in vapor-liquid equilibrium, the pressure in the tank is the saturation pressure, which is calculated using the Antoine equation:
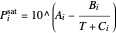 ,
,
where 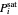 is the saturation pressure (bar),
is the saturation pressure (bar), 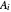 ,
, 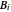 , and
, and 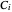 are Antoine constants, and
are Antoine constants, and 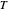 is temperature (K).
is temperature (K).
When only vapor is present, the ideal gas law is used to calculate the pressure 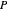 :
:
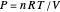 ,
,
where 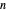 is the total moles (moles of vapor in this case),
is the total moles (moles of vapor in this case), 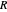 is the ideal gas constant ([L bar]/[mol K]), and
is the ideal gas constant ([L bar]/[mol K]), and 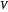 is the total volume (L).
is the total volume (L).
When the component is in VLE, the sum of the liquid 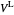 and vapor
and vapor 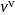 volumes equal the volume of the tank
volumes equal the volume of the tank 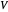 :
:
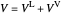 ,
,
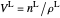 ,
,
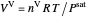 ,
,
and the total moles (moles injected) equals the sum of the liquid 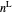 and vapor
and vapor 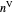 moles:
moles:
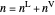 ,
,
where 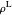 is the liquid molar density (mol/L).
is the liquid molar density (mol/L).
Solve 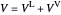 for
for 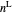 :
:
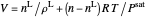 ,
,
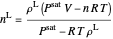 .
.
The screencast video at [1] explains how to use this Demonstration.
Reference
[1] Injecting a Liquid into an Evacuated Tank [Video]. (Dec 16, 2020) www.learncheme.com/simulations/thermodynamics/thermo-1/injecting-a-liquid-into-an-evacuated-tank.
Permanent Citation