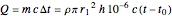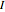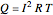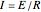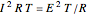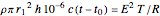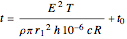Joule's Experiment and the First Law of Thermodynamics

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
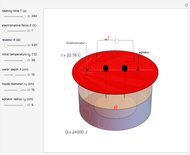
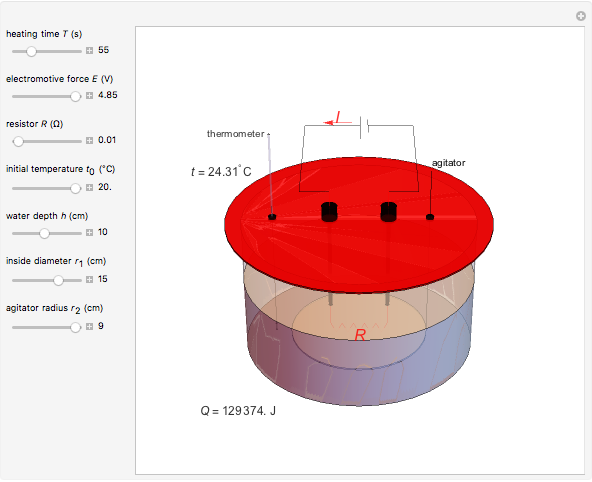
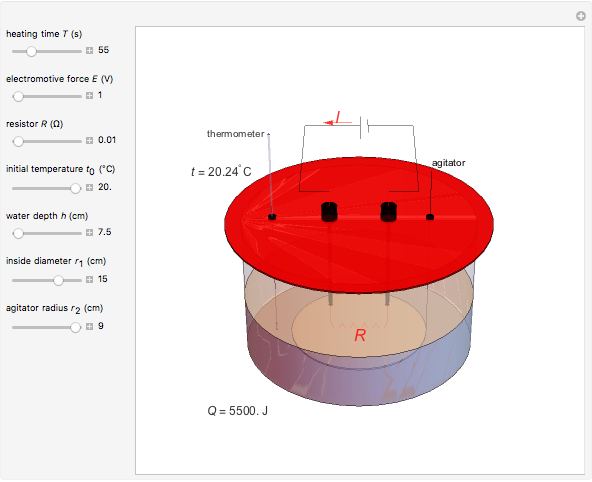
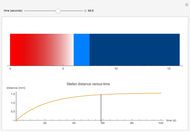
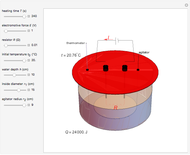
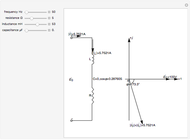
Joule's experiment demonstrated the validity of the first law of thermodynamics. Electrical or mechanical energy can be converted into thermal energy, but the total amount of energy is conserved. In this Demonstration, the amount of heat 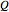 in joules generated in a circuit element of resistance
in joules generated in a circuit element of resistance 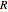 (immersion heater) is measured by a calorimeter. Assume that all the heat is absorbed by the water and measure the temperature increase from
(immersion heater) is measured by a calorimeter. Assume that all the heat is absorbed by the water and measure the temperature increase from 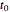 to
to  . Then
. Then
Contributed by: Anping Zeng (June 2015)
Open content licensed under CC BY-NC-SA
Snapshots
Details
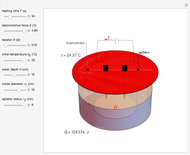
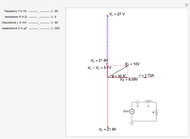
Snapshot 1: only heating time 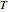 into 240 s
Snapshot 2: only voltage
into 240 s
Snapshot 2: only voltage 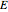 into 4.85 V
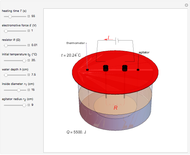
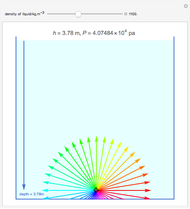
Snapshot 3: only depth of water into 7.5 cm
into 4.85 V
Snapshot 3: only depth of water into 7.5 cm
Permanent Citation