Mass Balance in the Haber Process

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
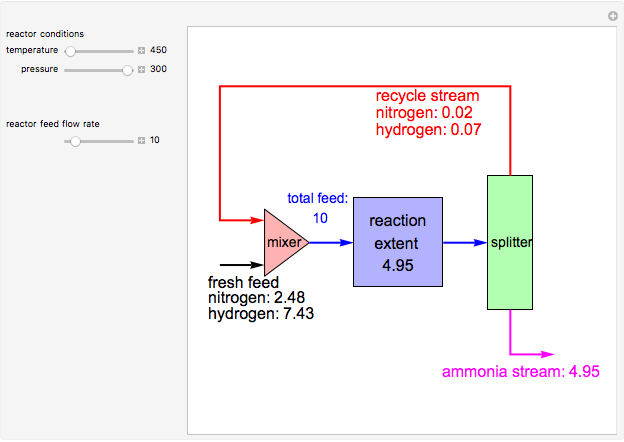
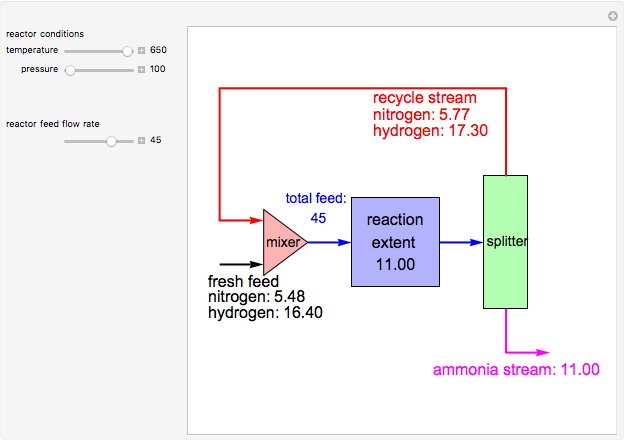
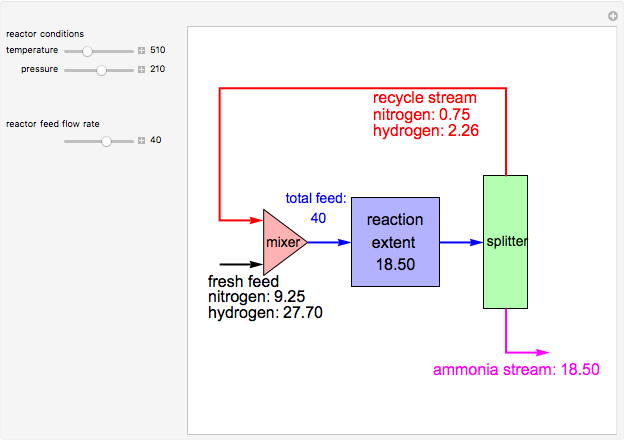
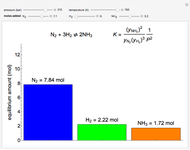
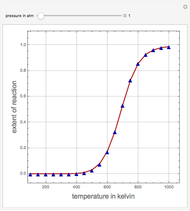
Consider the high-pressure synthesis of ammonia (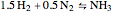 ), known as the Haber process. We use the Peng–Robinson equation of state and the reaction-coordinate method to compute the extent of reaction,
), known as the Haber process. We use the Peng–Robinson equation of state and the reaction-coordinate method to compute the extent of reaction,  in moles of
in moles of 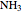 per unit time (e.g., hours), for user-set values of the reactor temperature
per unit time (e.g., hours), for user-set values of the reactor temperature 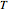 in degrees Kelvin and pressure
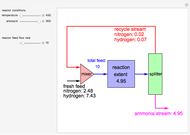
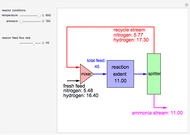
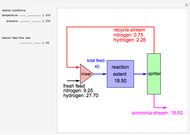
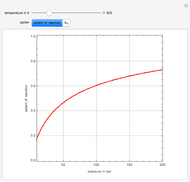
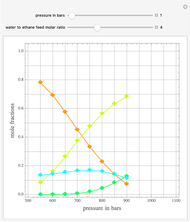
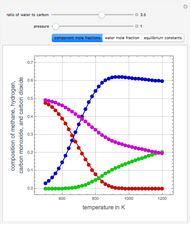
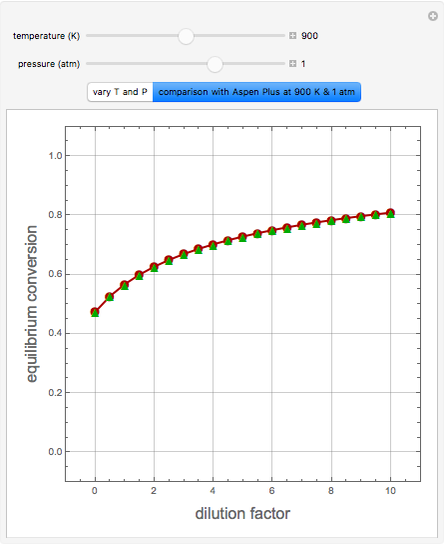
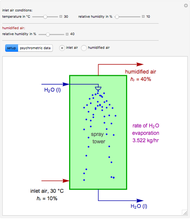
in degrees Kelvin and pressure 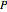 in bars. This Demonstration carries out the mass balance around the reactor and splitter in order to compute the amount of ammonia produced when chemical equilibrium is reached in the reactor and in the recycle stream. We assume that: (1) the splitter separates the product (i.e., ammonia) perfectly from the reactants (i.e., nitrogen and hydrogen); and (2) the fresh feed contains stoichiometric amounts of nitrogen and hydrogen. As expected from Le Chatelier's principle, the amount of ammonia produced is greater at low temperatures and high pressures.
in bars. This Demonstration carries out the mass balance around the reactor and splitter in order to compute the amount of ammonia produced when chemical equilibrium is reached in the reactor and in the recycle stream. We assume that: (1) the splitter separates the product (i.e., ammonia) perfectly from the reactants (i.e., nitrogen and hydrogen); and (2) the fresh feed contains stoichiometric amounts of nitrogen and hydrogen. As expected from Le Chatelier's principle, the amount of ammonia produced is greater at low temperatures and high pressures.
Contributed by: Housam Binous, Mohammad Mozahar Hossain, and Ahmed Bellagi (October 2015)
Open content licensed under CC BY-NC-SA
Snapshots
Details
detailSectionParagraphPermanent Citation
"Mass Balance in the Haber Process"
http://demonstrations.wolfram.com/MassBalanceInTheHaberProcess/
Wolfram Demonstrations Project
Published: October 19 2015