Multiple States in an Isothermal Continuous Stirred-Tank Reactor

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
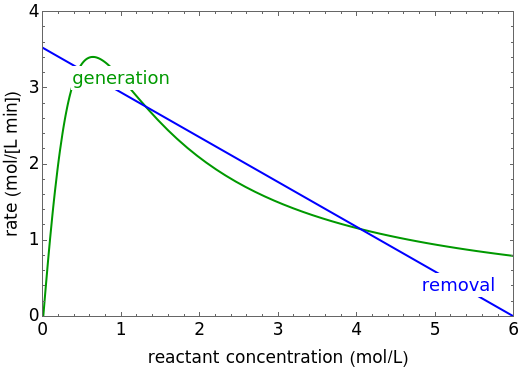
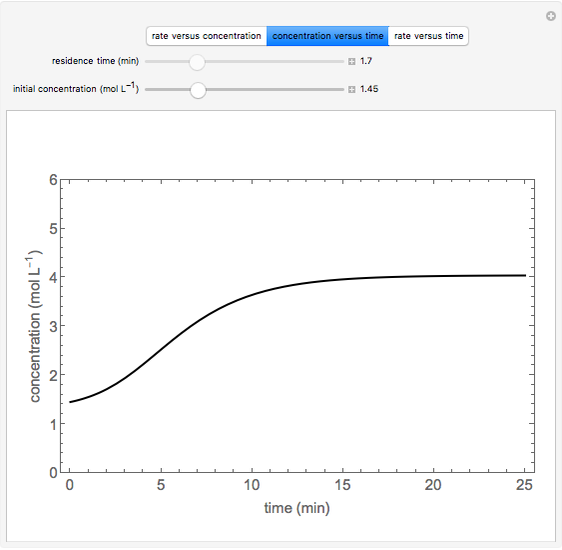
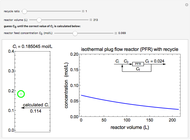
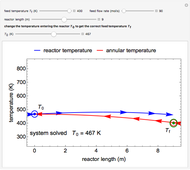
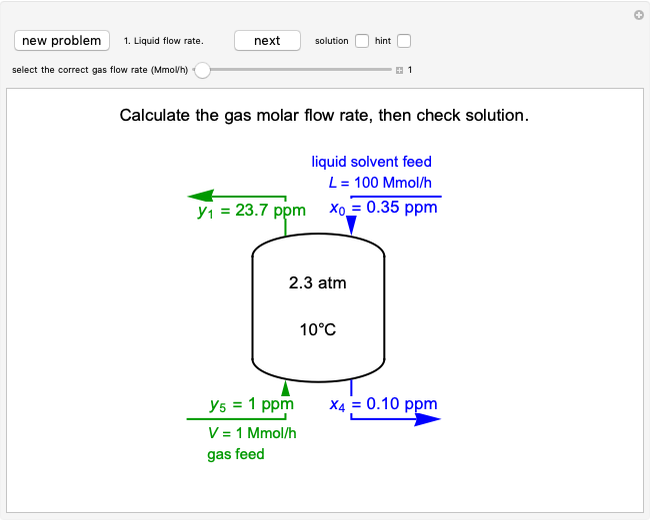
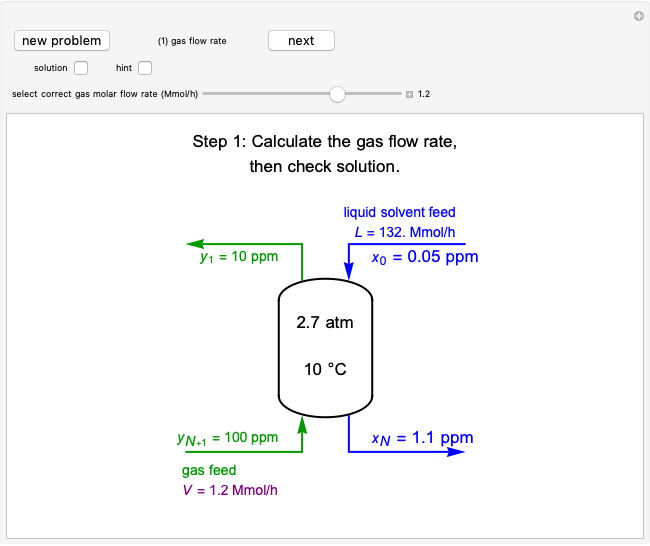
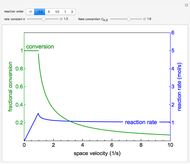
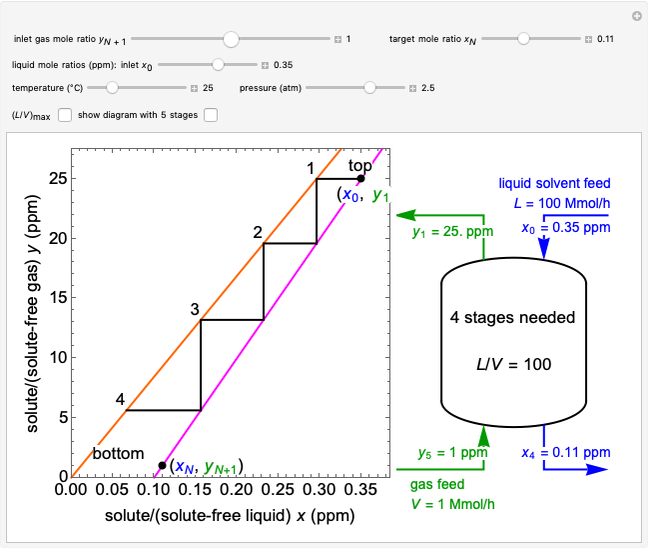
An irreversible reaction 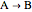 is carried out in an isothermal, continuous stirred-tank reactor (CSTR). The green curve in the "rate vs. concentration" plot shows the non-linear dependence of the reaction rate (
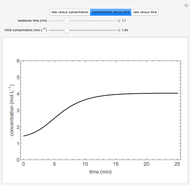
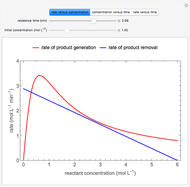
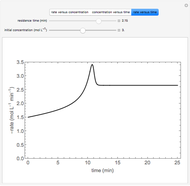
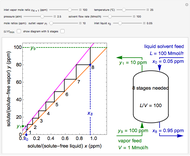
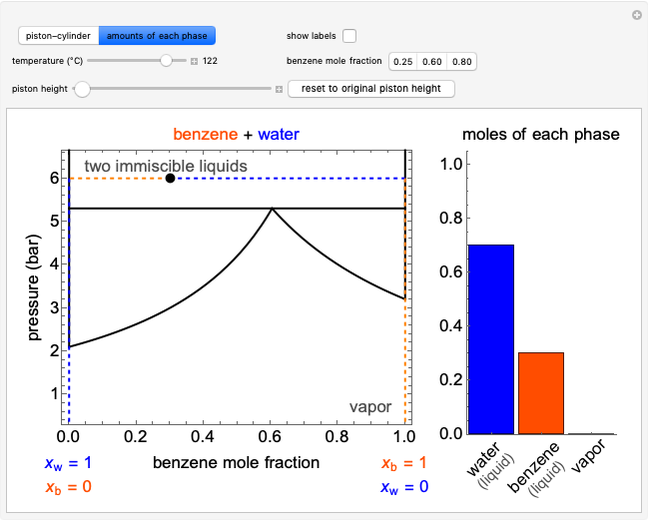
is carried out in an isothermal, continuous stirred-tank reactor (CSTR). The green curve in the "rate vs. concentration" plot shows the non-linear dependence of the reaction rate (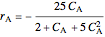 ). The blue line is the CSTR mass balance, and the intersections of the green curve and the blue line are the steady-state solutions to the mass balance. When three solutions are obtained, only two are stable. Changing the residence time with the slider changes the steady-state solutions, and at high and low residence times, only one solution exists. The "concentration vs. time" and "rate vs. time" plots show how the concentration and rate approach steady state when the initial reactant concentration in the reactor, which can be changed with the slider, is not the steady-state concentration. When the reactor has three steady-state solutions, if the initial reactant concentration is above the concentration for the middle steady state, then the reactant concentration in the reactor approaches the higher steady-state reactant concentration. Otherwise, it approaches the lower steady-state reactant concentration. The feed concentration to the reactor is 6.0 mol/L.
). The blue line is the CSTR mass balance, and the intersections of the green curve and the blue line are the steady-state solutions to the mass balance. When three solutions are obtained, only two are stable. Changing the residence time with the slider changes the steady-state solutions, and at high and low residence times, only one solution exists. The "concentration vs. time" and "rate vs. time" plots show how the concentration and rate approach steady state when the initial reactant concentration in the reactor, which can be changed with the slider, is not the steady-state concentration. When the reactor has three steady-state solutions, if the initial reactant concentration is above the concentration for the middle steady state, then the reactant concentration in the reactor approaches the higher steady-state reactant concentration. Otherwise, it approaches the lower steady-state reactant concentration. The feed concentration to the reactor is 6.0 mol/L.
Contributed by: Rachael L. Baumann (November 2013)
Additional contributions by: John L. Falconer and Nick Bongiardina
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Rate equations:
τ = 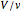 ,
,
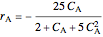 ,
,
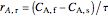 ,
,
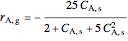 ,
,
where  is residence time (min),
is residence time (min), 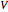 is reactor volume (L),
is reactor volume (L),  is the volumetric flow rate (L/min),
is the volumetric flow rate (L/min), 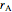 is reaction rate (mol/[L min]),
is reaction rate (mol/[L min]), 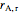 is the steady-state rate (mol/[L min]),
is the steady-state rate (mol/[L min]), 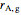 is the transient rate expression (mol/[L min]),
is the transient rate expression (mol/[L min]), 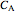 is the concentration of reactant
is the concentration of reactant 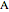 (mol/L), and
(mol/L), and 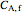 and
and 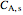 are the feed and steady-state concentrations of reactant
are the feed and steady-state concentrations of reactant 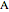 (mol/L).
(mol/L).
Mole balance:
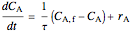 ,
,
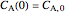 ,
,
where  is the initial concentration of
is the initial concentration of 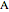 in the reactor (mol/L).
in the reactor (mol/L).
The screencast video at [1] shows how to use this Demonstration.
Reference
[1] Multiple States in an Isothermal Continuous Stirred-Tank Reactor. www.colorado.edu/learncheme/kinetics/MultipleStatesIsothermalCSTR.html.
Permanent Citation