Oxygen Transport by Hemoglobin and Myoglobin

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
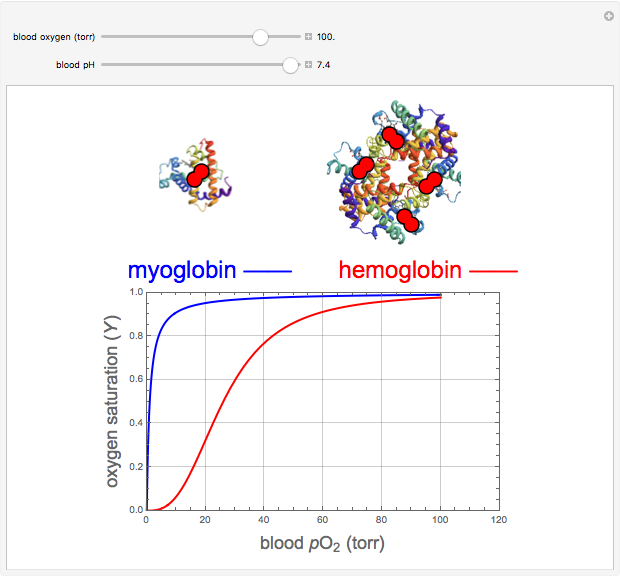
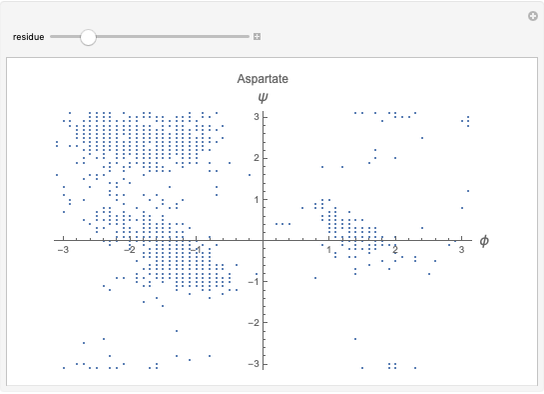
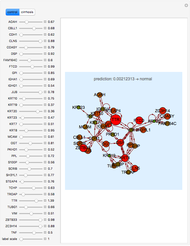
Hemoglobin, the essential component of red blood cells (erythrocytes), transports oxygen (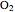 ) through the bloodstream from the lungs to all the tissues of the body. Hemoglobin also carries carbon dioxide (
) through the bloodstream from the lungs to all the tissues of the body. Hemoglobin also carries carbon dioxide (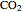 ) back to the lungs to complete the process of respiration. Vertebrate hemoglobin is a nearly spherical protein molecule consisting of an array of four globin polypeptide chains, each containing a heme group, which is the oxygen-binding site. Its molecular weight is approximately 64,500 daltons. The oxygen uptake of hemoglobin exhibits cooperativity, such that each
) back to the lungs to complete the process of respiration. Vertebrate hemoglobin is a nearly spherical protein molecule consisting of an array of four globin polypeptide chains, each containing a heme group, which is the oxygen-binding site. Its molecular weight is approximately 64,500 daltons. The oxygen uptake of hemoglobin exhibits cooperativity, such that each 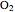 successively increases the affinity of the molecule for adding another
successively increases the affinity of the molecule for adding another 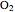 , up to four. The saturation
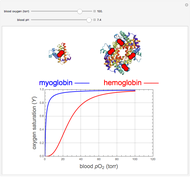
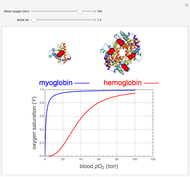
, up to four. The saturation 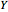 is a measure of the fractional occupancy of the oxygen-binding sites. It increases as a sigmoid-shaped function of the partial pressure of
is a measure of the fractional occupancy of the oxygen-binding sites. It increases as a sigmoid-shaped function of the partial pressure of 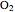 in its immediate environment. In the alveoli of the lungs,
in its immediate environment. In the alveoli of the lungs, 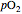 is approximately 100 torr.
is approximately 100 torr.
Contributed by: S. M. Blinder (April 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
The binding of oxygen to myoglobin is described by the equilibrium 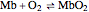 . The saturation can be defined by
. The saturation can be defined by 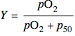 , where
, where 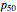 is the partial pressure for 50% saturation, about 1 torr for myoglobin. For hemoglobin, the equilibrium takes the form
is the partial pressure for 50% saturation, about 1 torr for myoglobin. For hemoglobin, the equilibrium takes the form 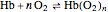 ,
,  to
to  . Here
. Here 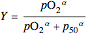 , where
, where 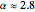 to account for the cooperativity of
to account for the cooperativity of 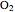 binding and
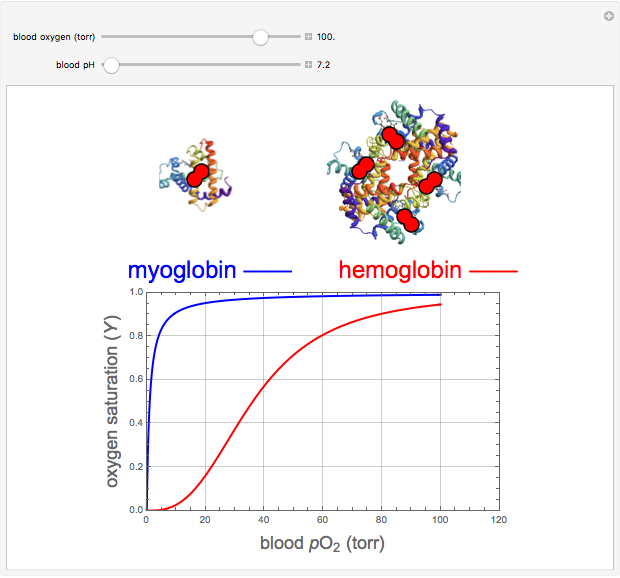
binding and 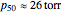 at pH 7.4 but slightly higher as the pH decreases.
at pH 7.4 but slightly higher as the pH decreases.
The central part of the heme group has this structure: 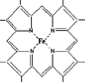 . The
. The 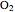 complexes with the iron atom.
complexes with the iron atom.
Reference: L. Stryer, Chap. 7, Molecular Design of Life, New York: W. H. Freeman, 1989.
Permanent Citation