Partial Molar Enthalpy

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
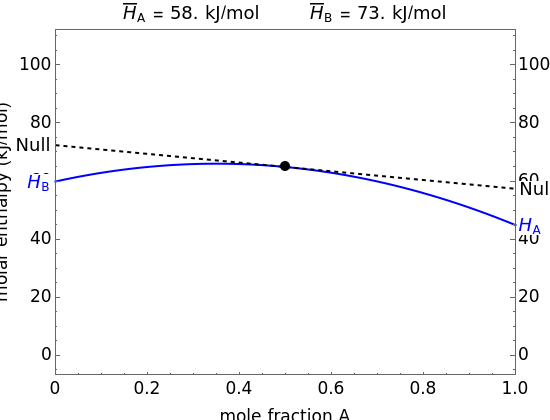
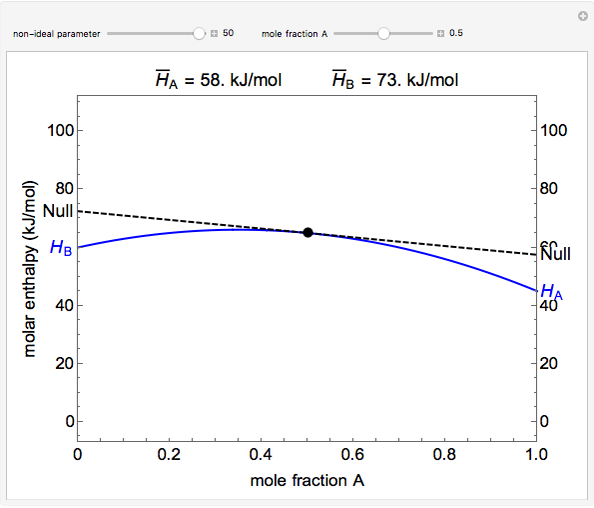
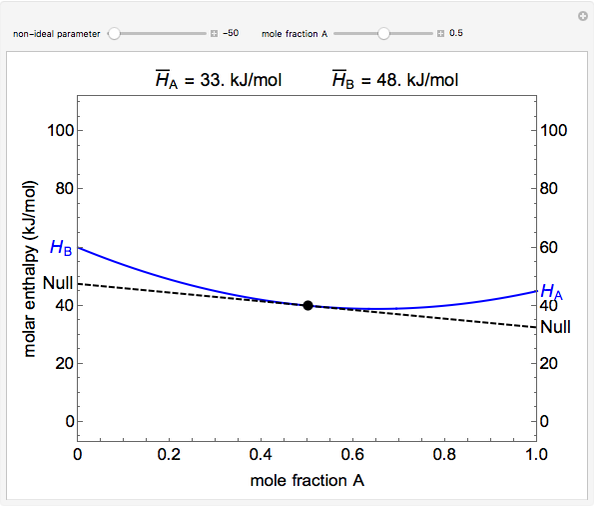
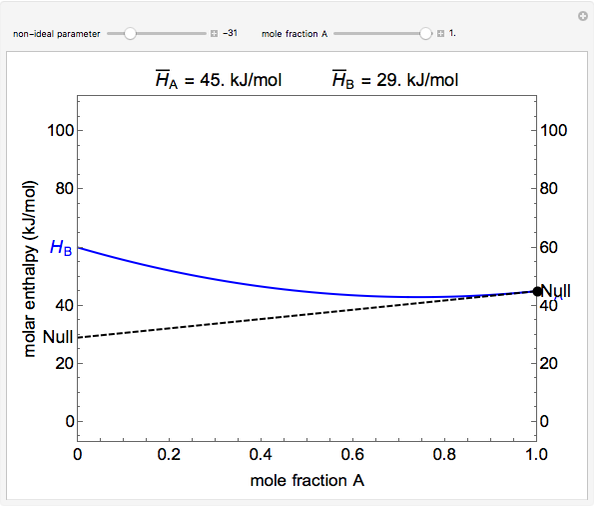
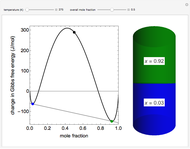
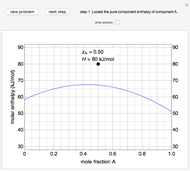
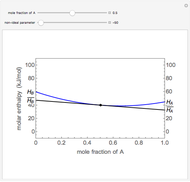
The molar enthalpy of a binary mixture (blue curve) of  and
and 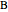 is plotted as a function of the mole fraction of component
is plotted as a function of the mole fraction of component 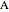 . The end points of the molar enthalpy are the pure-component enthalpies
. The end points of the molar enthalpy are the pure-component enthalpies 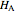 and
and 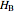 . The partial molar enthalpies
. The partial molar enthalpies 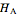 and
and 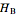 are obtained by drawing a tangent line (black, dashed) at the black point, which indicates the mole fraction of the solution. The intersections of this tangent with the
are obtained by drawing a tangent line (black, dashed) at the black point, which indicates the mole fraction of the solution. The intersections of this tangent with the 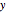 -axis at
-axis at 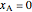 and
and 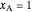 correspond to
correspond to 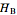 and
and 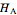 , respectively. You can change the mole fraction of
, respectively. You can change the mole fraction of 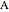 in the mixture and the non-ideal parameter, which represents deviation from an ideal solution, with sliders. For an ideal solution the non-ideal parameter is zero, and the enthalpy of the mixture is a linear function of the molar enthalpies of the pure components.
in the mixture and the non-ideal parameter, which represents deviation from an ideal solution, with sliders. For an ideal solution the non-ideal parameter is zero, and the enthalpy of the mixture is a linear function of the molar enthalpies of the pure components.
Contributed by: Simon M. Lane and Rachael L. Baumann (April 2014)
With additional contributions by: John L. Falconer and Nick Bongiardina
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Details
The molar enthalpy 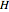 is:
is:
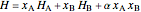 ,
,
where 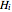 is the enthalpy (kJ/mol),
is the enthalpy (kJ/mol), 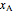 and
and 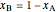 are the compositions of
are the compositions of 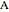 and
and 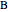 , and
, and  is a non-ideal parameter.
is a non-ideal parameter.
The partial molar enthalpy 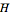 is represented by a line tangent to
is represented by a line tangent to 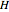 at composition of the mixture
at composition of the mixture 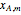 :
:
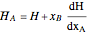
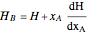
Where 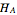 is the partial molar enthalpy of component
is the partial molar enthalpy of component 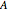 and
and 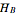 is the partial molar enthalpy of component
is the partial molar enthalpy of component 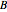 . A screencast video at [1] shows how to use this Demonstration, and a screencast at [2] presents an example.
. A screencast video at [1] shows how to use this Demonstration, and a screencast at [2] presents an example.
References
[1] Partial Molar Enthalpy. http://www.learncheme.com/simulations/thermodynamics/thermo-2/partial-molar-h
[2] Partial Molar Properties: Binary Solutions [Video]. (Apr 5, 2012) www.youtube.com/watch?v=TFmIPEG_X3A.
Snapshots
Permanent Citation