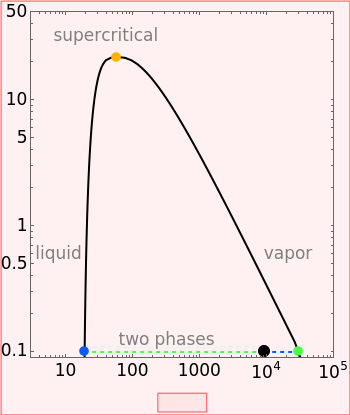
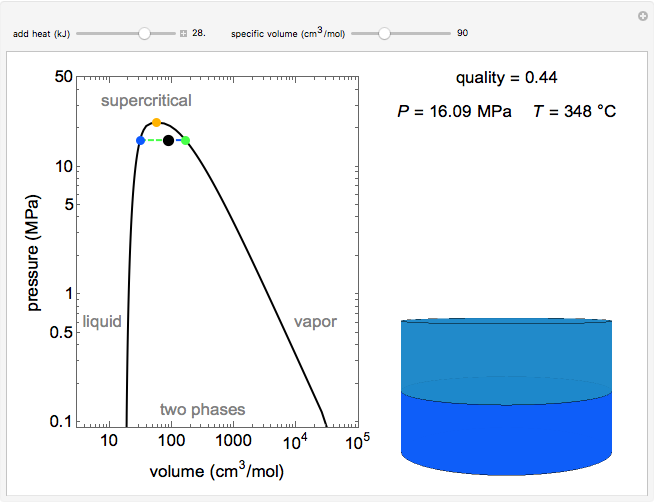
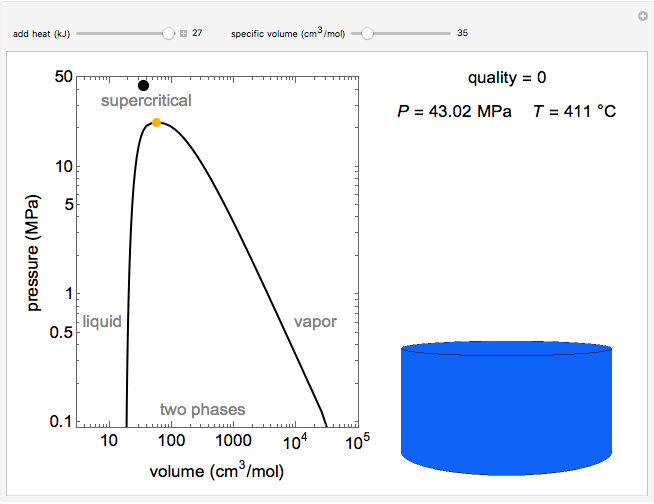
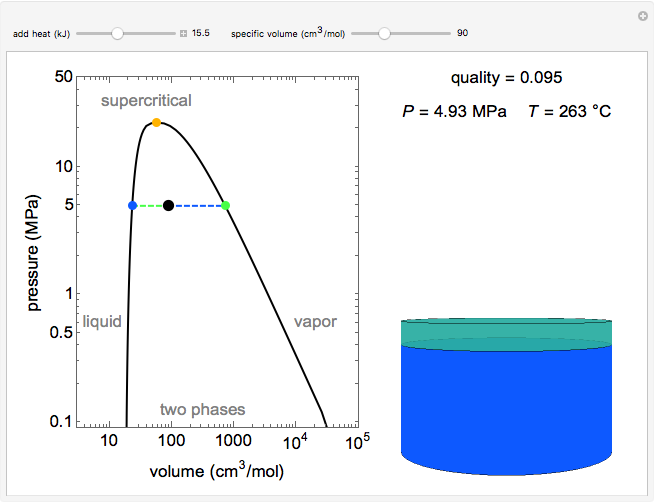
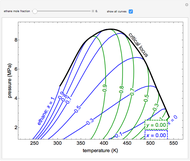
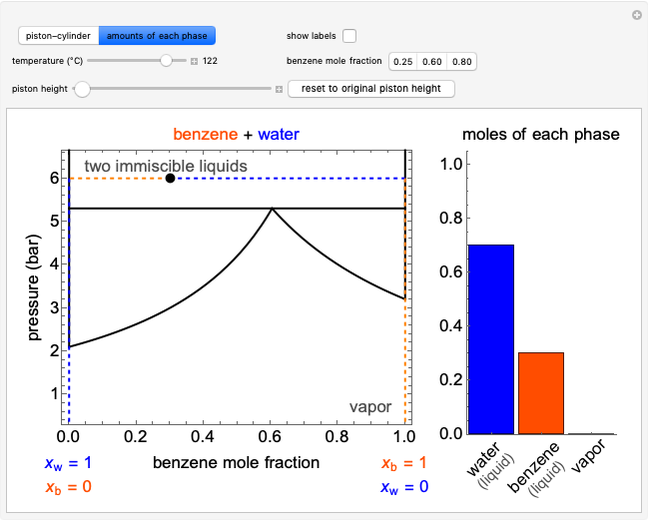
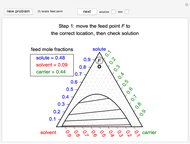
Pressure-Volume Diagram for Heating a Vapor-Liquid Mixture at a Constant Volume
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
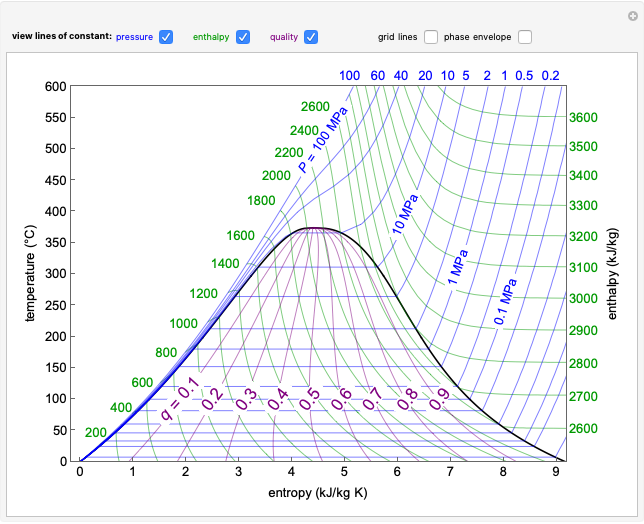
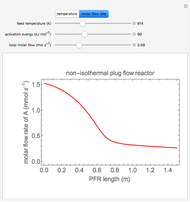
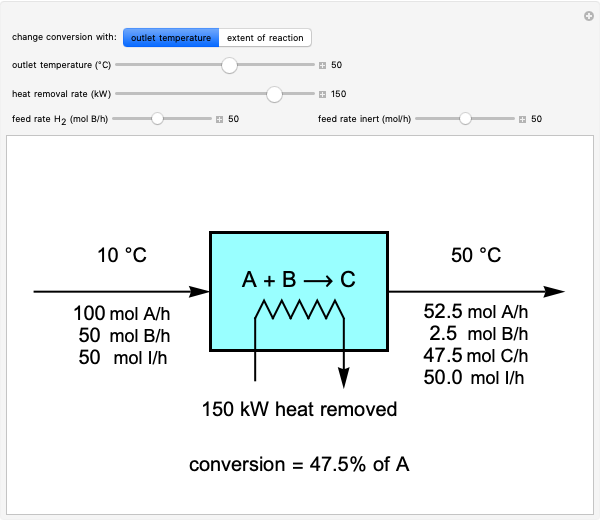
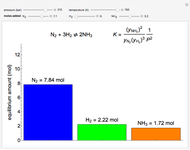
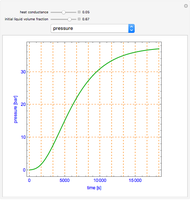
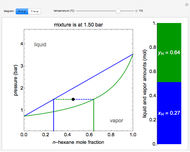
This Demonstration shows the phase behavior of 1 mol of water on a log pressure versus a log volume (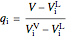 -
-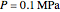 ) phase diagram.
) phase diagram.
Contributed by: Adam J. Johnston and Rachael L. Baumann (September 2017)
Additional contributions by: John L. Falconer
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
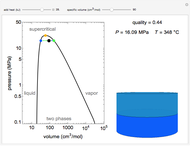
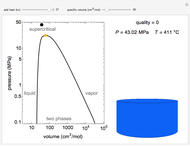
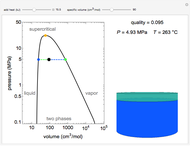
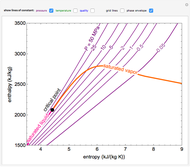
Snapshots
Details
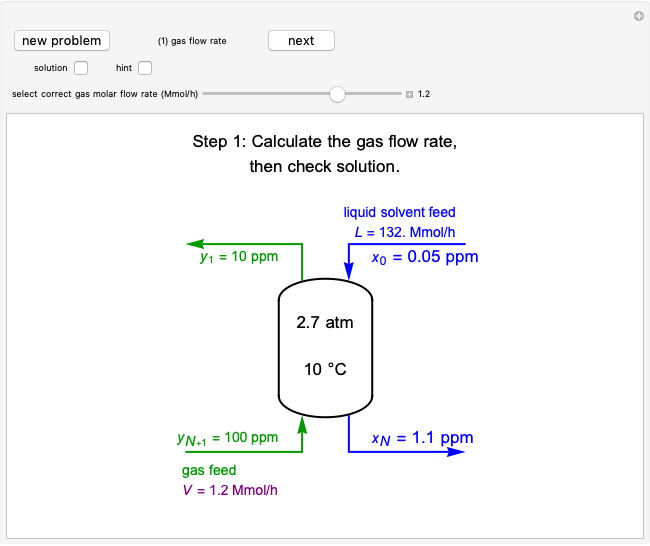
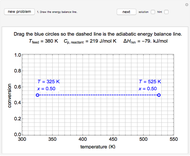
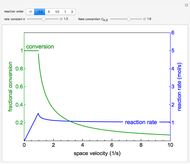
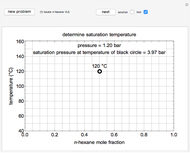
Mass and energy balances are used to calculate the pressure and quality when two phases are present: