Rankine Cycle

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
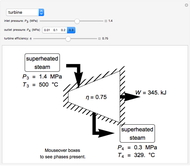
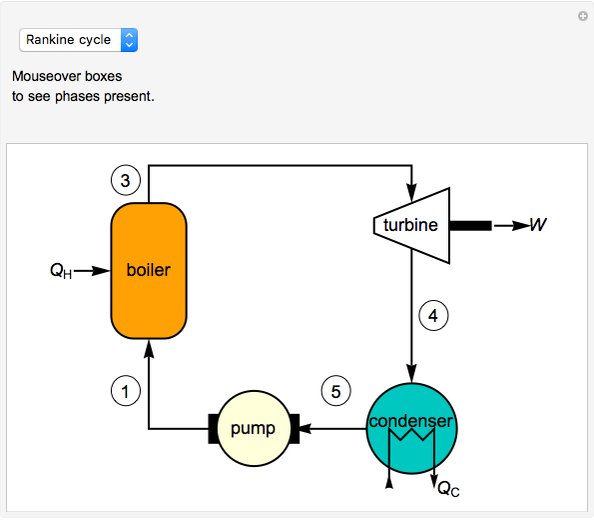
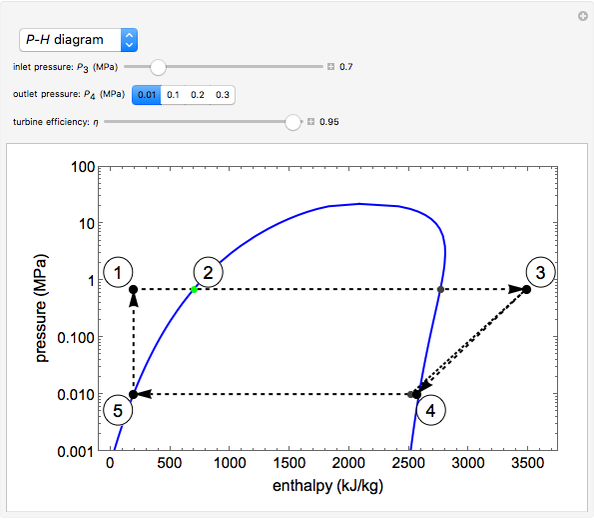
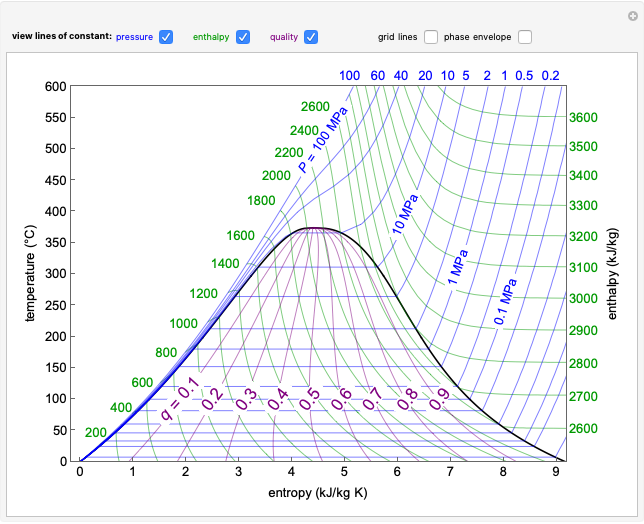
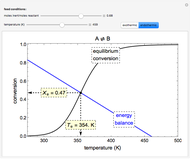
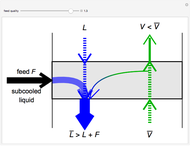
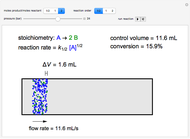
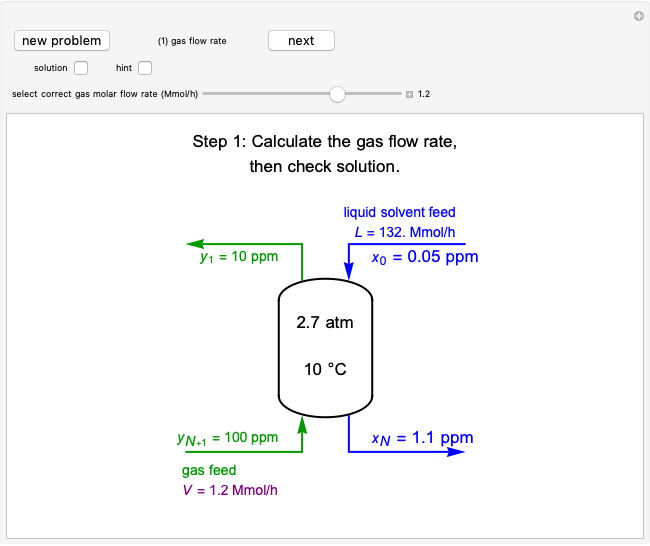
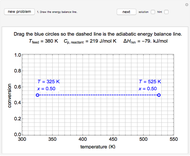
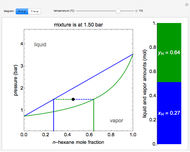
In a Rankine cycle, high-pressure liquid water (1) enters a boiler where it is heated to saturation temperature (2), vaporized, and superheated (3). The superheated steam is fed to a turbine, where it expands and generates mechanical work. The steam exits the turbine at a lower pressure and temperature as either superheated steam or steam with a vapor quality (4). After the steam is condensed (5), a pump compresses the liquid water to high pressure (1). Select " diagram" to see the cycle on a pressure-enthalpy diagram. Use sliders to select the inlet pressure to the turbine
diagram" to see the cycle on a pressure-enthalpy diagram. Use sliders to select the inlet pressure to the turbine 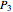 and one of four outlet pressures
and one of four outlet pressures 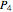 . Use a slider to vary the turbine efficiency
. Use a slider to vary the turbine efficiency 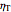 , which is the ratio of work produced by the turbine to the work produced by a reversible turbine between the same inlet and outlet pressures. The irreversible turbine pathway (
, which is the ratio of work produced by the turbine to the work produced by a reversible turbine between the same inlet and outlet pressures. The irreversible turbine pathway (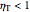 ) is the dashed black line on the
) is the dashed black line on the 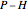 diagram; the reversible turbine pathway is the orange dashed line. Select "Rankine cycle" to view a schematic of the cycle, and select "turbine" to show the inlet and outlet conditions for the turbine and the work generated.
diagram; the reversible turbine pathway is the orange dashed line. Select "Rankine cycle" to view a schematic of the cycle, and select "turbine" to show the inlet and outlet conditions for the turbine and the work generated.
Contributed by: Rachael L. Baumann (November 2014)
Additional contributions by: John L. Falconer and Nick Bongiardina
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
The inlet temperature to the turbine, 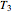 (°C) is set; you can vary the inlet
(°C) is set; you can vary the inlet 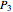 and outlet
and outlet 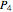 pressures (MPa). From these conditions the enthalpy
pressures (MPa). From these conditions the enthalpy 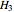 (kJ/kg) and entropy
(kJ/kg) and entropy 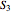 (kJ/[kg K]) are obtained from the superheated steam tables.
(kJ/[kg K]) are obtained from the superheated steam tables.
The superscript  denotes reversibility. A reversible steam turbine is isentropic, so
denotes reversibility. A reversible steam turbine is isentropic, so  , where
, where 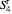 is the entropy of the steam exiting a reversible turbine.
is the entropy of the steam exiting a reversible turbine.
If 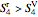 at
at 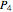 , then the reversible outlet state is superheated.
, then the reversible outlet state is superheated.
If 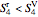 at
at 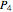 , then the outlet steam is at saturation temperature and consists of liquid and vapor. The quality of the steam is:
, then the outlet steam is at saturation temperature and consists of liquid and vapor. The quality of the steam is:
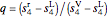 ,
,
where  is the fraction of vapor (quality), and the superscripts
is the fraction of vapor (quality), and the superscripts  and
and 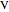 refer to saturated liquid and saturated vapor.
refer to saturated liquid and saturated vapor.
Depending on the quality of the exiting steam, 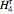 is obtained from either the saturated steam tables or the superheated steam tables.
is obtained from either the saturated steam tables or the superheated steam tables.
If 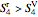 ,
,  and
and 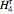 is found in the superheated steam tables at
is found in the superheated steam tables at 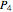 and
and 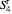 .
.
If 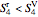 ,
, 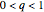 and
and 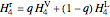 .
.
The change in enthalpy for the reversible turbine 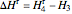 is the work for the reversible process.
is the work for the reversible process.
The turbine efficiency 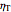 indicates the turbine irreversibility and is the ratio of irreversible work to reversible work. For the irreversible turbine:
indicates the turbine irreversibility and is the ratio of irreversible work to reversible work. For the irreversible turbine:
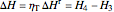 .
.
The cycle efficiency is:
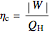 ,
,
where 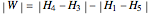 is the net work (kJ/kg), and
is the net work (kJ/kg), and 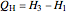 is heat added (kJ/kg).
is heat added (kJ/kg).
The screencast video at [1] shows how to use this Demonstration, and the video at [2] explains the cycle.
References
[1] Rankine Cycle. www.colorado.edu/learncheme/thermodynamics/RankineCycle.html.
[2] Power Cycle Introduction [Video]. (Mar 15, 2012) www.youtube.com/watch?v=BA77fu3zAbs.
Permanent Citation