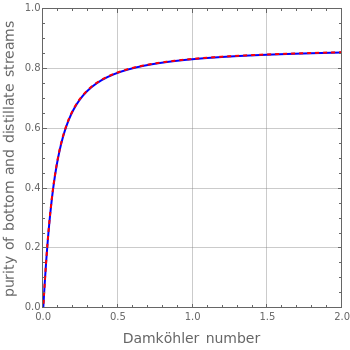
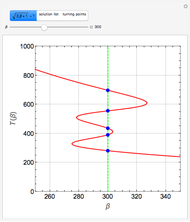
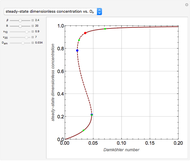
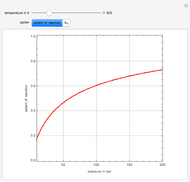
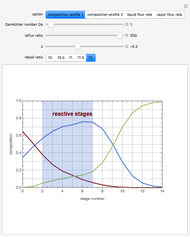
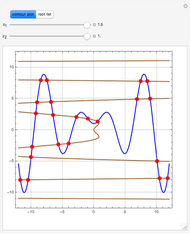
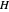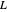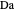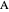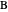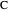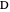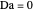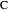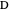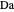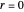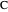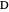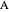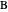Reactive Distillation Using Arc Length Continuation

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
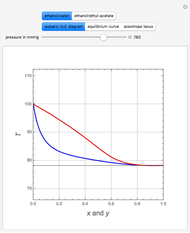
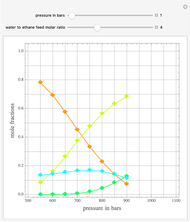
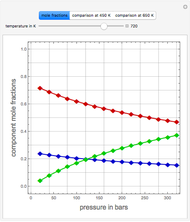
Consider a mixture of four components 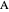 ,
, 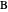 ,
, 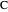 , and
, and 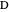 with relative volatilities
with relative volatilities 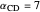 ,
, 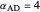 , and
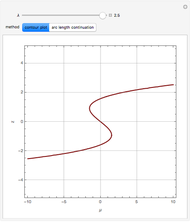
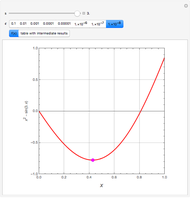
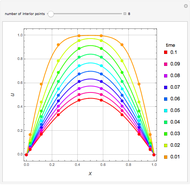
, and 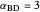 . This mixture is subject to an equilibrium-limited chemical reaction
. This mixture is subject to an equilibrium-limited chemical reaction 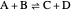 with reaction rate
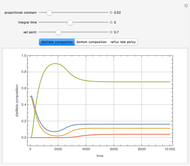
with reaction rate 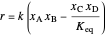 , where the user can set the value of the equilibrium constant
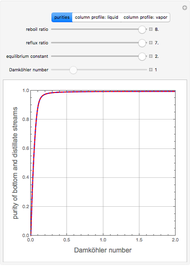
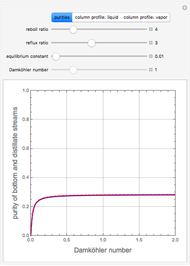
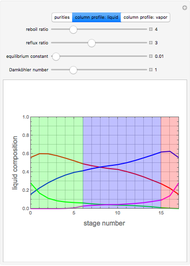
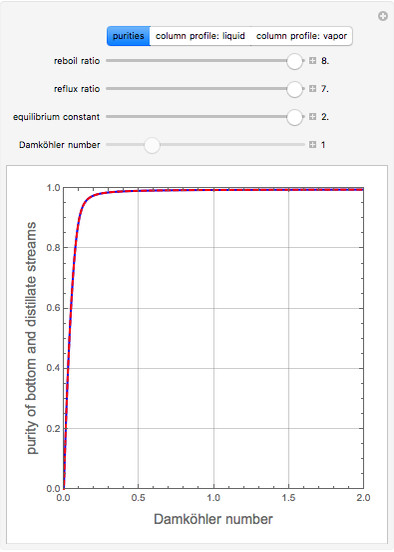
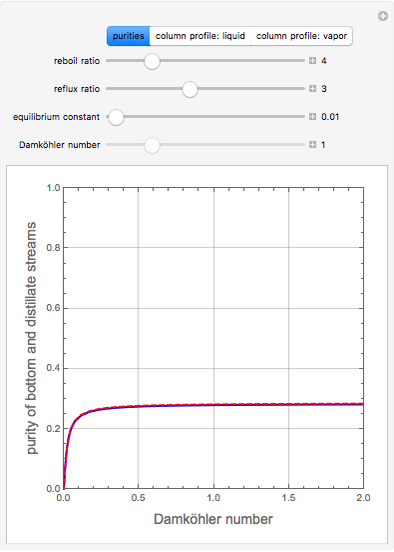
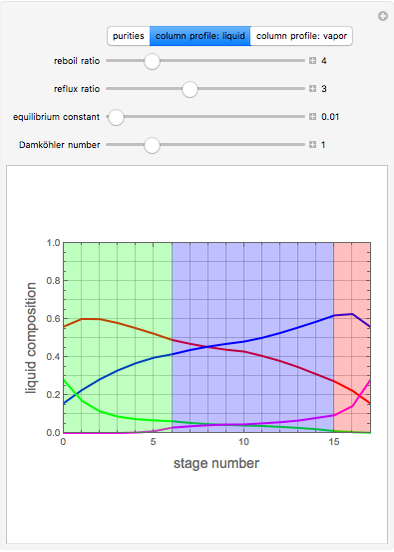
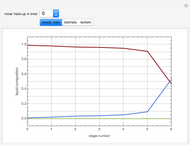
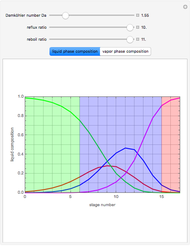
, where the user can set the value of the equilibrium constant 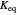 . This mixture is fed to a reactive distillation column such that the total number of plates is 17. The feed stage location is stage number 10, the reactive stages are from stage 6 to stage 14, and the feed composition is equimolar in
. This mixture is fed to a reactive distillation column such that the total number of plates is 17. The feed stage location is stage number 10, the reactive stages are from stage 6 to stage 14, and the feed composition is equimolar in 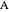 and
and 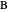 (i.e. the feed is composed of 50 mole %
(i.e. the feed is composed of 50 mole % 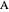 and 50 mole %
and 50 mole % 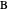 ). The feed, a saturated liquid, has a flow rate of 100 kmol/hr. For simplicity, constant molal overflow (CMO) is assumed and heat effects are neglected.
). The feed, a saturated liquid, has a flow rate of 100 kmol/hr. For simplicity, constant molal overflow (CMO) is assumed and heat effects are neglected.
Contributed by: Housam Binous, Ahmed Bellagi, and Brian G. Higgins (December 2013)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Permanent Citation