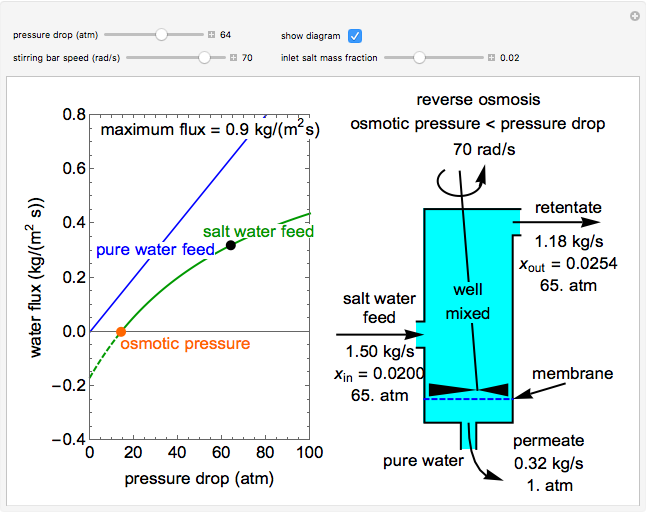
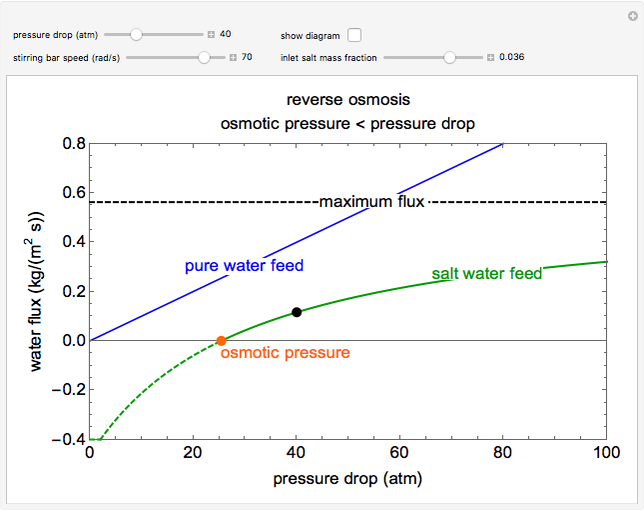
Reverse Osmosis

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
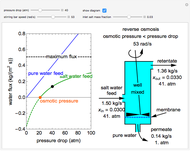
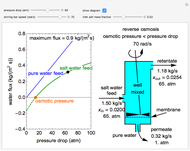
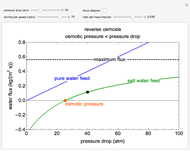
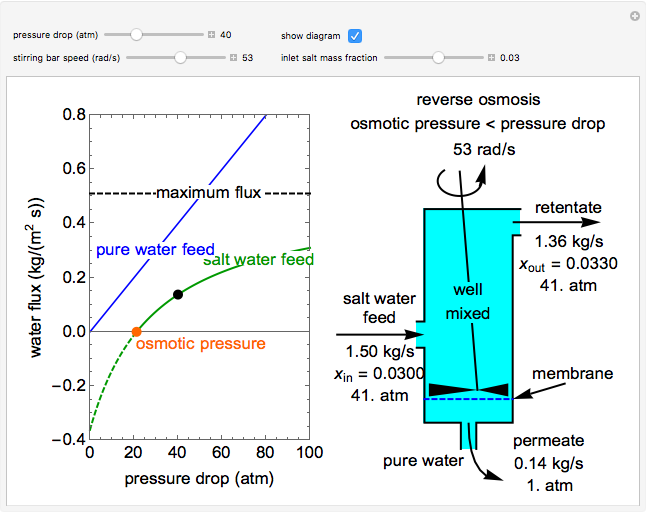
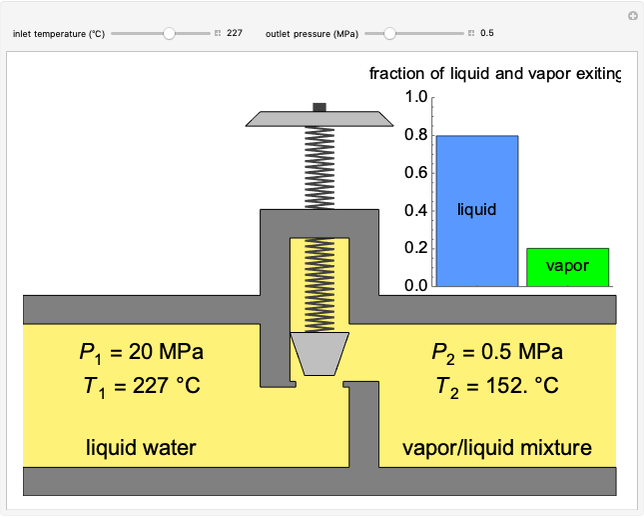
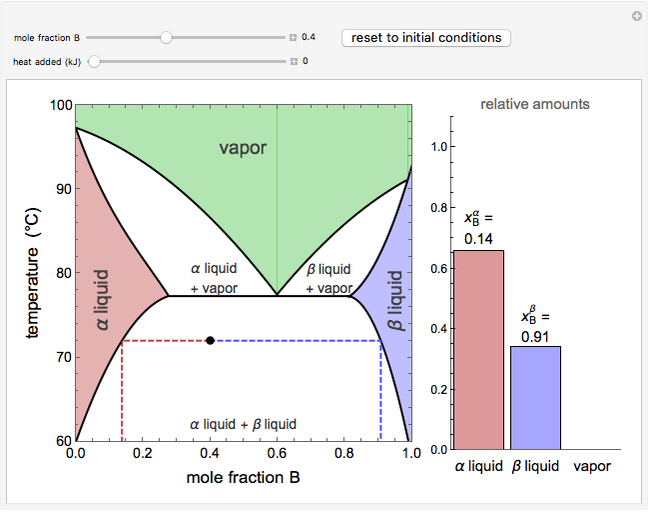
This Demonstration models a reverse osmosis process in which salt water at high pressure is fed to a chamber with a membrane that is only permeable to water. The outlet permeate stream is pure water at low pressure, and the retentate stream is water enriched in salt.
[more]
Contributed by: Muqbil Alkhalaf and Rachael L. Baumann (September 2007)
Additional contributions by: John L. Falconer and Neil Hendren
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Details
First, solve for the mass transfer coefficient using the equation:
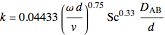 ,
,
where  is the mass transfer coefficient,
is the mass transfer coefficient,
 is the rotational speed of the stirring bar (rad/s),
is the rotational speed of the stirring bar (rad/s),
 is the tank diameter (m),
is the tank diameter (m),
 is kinematic viscosity (
is kinematic viscosity (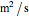 ),
),
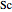 is the Schmidt number (dimensionless) and
is the Schmidt number (dimensionless) and
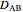 is the diffusivity of the solute in water (
is the diffusivity of the solute in water (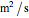 ).
).
Then solve for the maximum solvent flux:
 ,
,
where 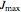 is maximum water flux through the membrane (
is maximum water flux through the membrane (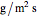 ) and
) and  is the density of water (
is the density of water (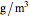 ).
).
Solve for the solvent flux:
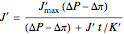 ,
,
where 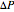 is mechanical pressure (atm),
is mechanical pressure (atm),
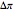 is osmotic pressure (atm) and
is osmotic pressure (atm) and
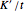 is permeability of the membrane to the solvent (
is permeability of the membrane to the solvent (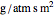 ).
).
A material balance is done for salt across the system to get the value for the mass fraction of salt in the outlet stream, which is the same as the retentate mass fraction inside the tank (given the tank is well mixed):
where 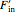 is inlet feed (kg/s),
is inlet feed (kg/s), 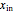 is the mole fraction of salt in the inlet stream, and
is the mole fraction of salt in the inlet stream, and 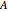 is the area of the membrane (
is the area of the membrane (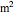 ).
).
To find how much of the feed exits as the permeate, an overall mass balance is done:
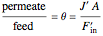 ,
,
where  is the fraction of the feed that is purified and exits as the permeate, i.e., cut
is the fraction of the feed that is purified and exits as the permeate, i.e., cut  (dimensionless).
(dimensionless).
Snapshots
Permanent Citation