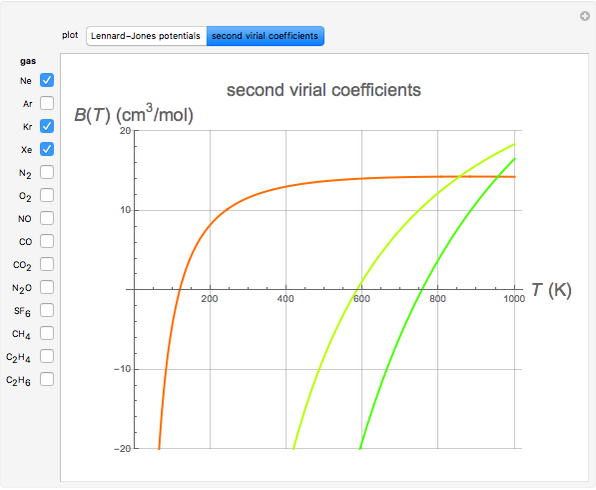
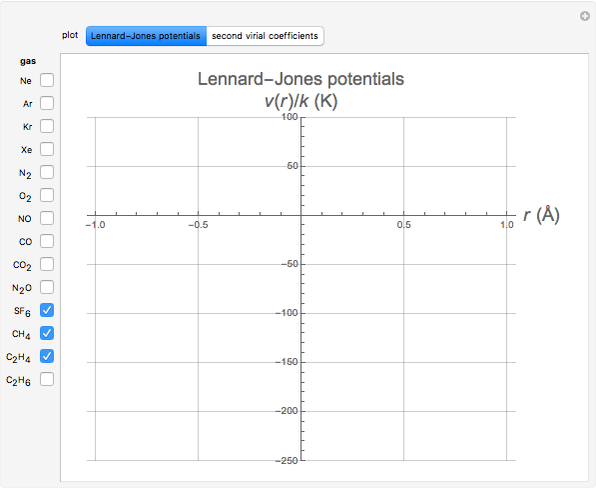
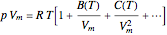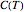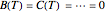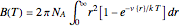Second Virial Coefficients Using the Lennard-Jones Potential

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
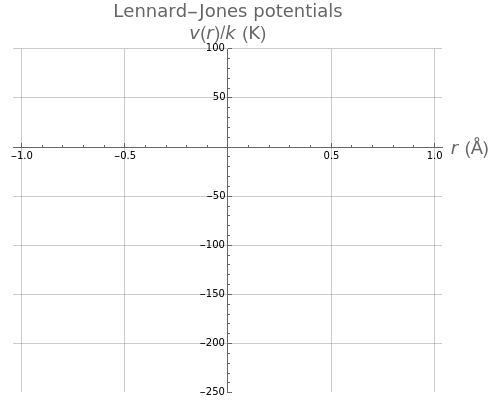
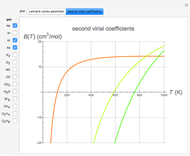
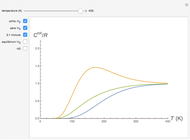
The Lennard–Jones (L–J) 6-12 potential, 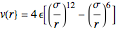 , has been extensively employed for the computation of virial coefficients and transport properties of fluids. Here
, has been extensively employed for the computation of virial coefficients and transport properties of fluids. Here  and
and  are empirical parameters for each substance. The depth of the potential well is equal to
are empirical parameters for each substance. The depth of the potential well is equal to 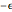 , while the collision diameter is approximated by
, while the collision diameter is approximated by  . The equilibrium intermolecular separation is then given by
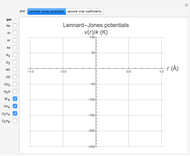
. The equilibrium intermolecular separation is then given by 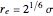 . In the first part of this Demonstration, L–J potentials are plotted for a number of common gases.
. In the first part of this Demonstration, L–J potentials are plotted for a number of common gases.
Contributed by: S. M. Blinder (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
The integral for 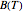 cannot be evaluated analytically, but an accurate approximation can be based on the expansion
cannot be evaluated analytically, but an accurate approximation can be based on the expansion 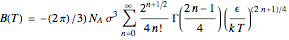 . It suffices to take just the first few terms of the series.
. It suffices to take just the first few terms of the series.
References
[1] S. M. Blinder, Advanced Physical Chemistry, London: Macmillan, 1969, pp. 491–496.
[2] J. O. Hirschfelder, C. F. Curtiss, and R. B. Bird, Molecular Theory of Gases and Liquids, New York: Wiley, 1954.
Permanent Citation