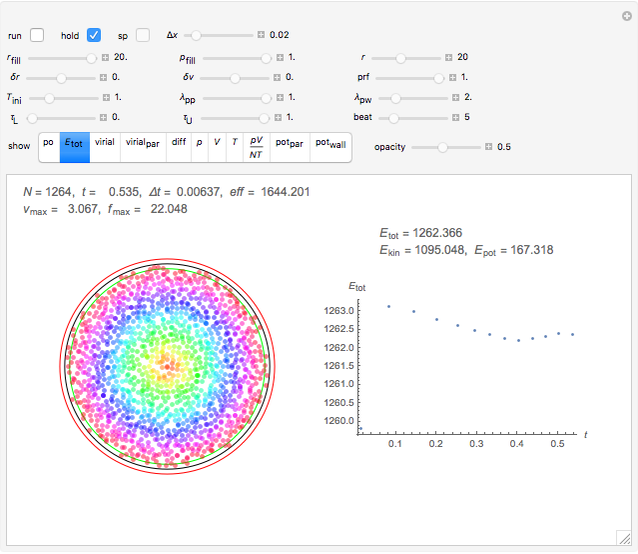
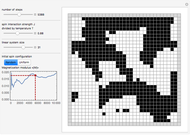
Simulating Real Gases in 2D

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
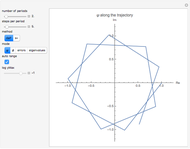
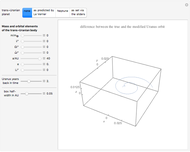
This Demonstration simulates the effects of intermolecular forces on the pressure and temperature of  gas particles enclosed in a spherical container. The interparticle forces are constructed to have a finite range. The time-stepping algorithm that is employed takes advantage of this to reduce the time complexity, which for pairwise forces normally is
gas particles enclosed in a spherical container. The interparticle forces are constructed to have a finite range. The time-stepping algorithm that is employed takes advantage of this to reduce the time complexity, which for pairwise forces normally is 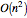 , to the much more manageable
, to the much more manageable 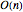 .
.
Contributed by: Ulrich Mutze (December 2016)
Open content licensed under CC BY-NC-SA
Snapshots
Details
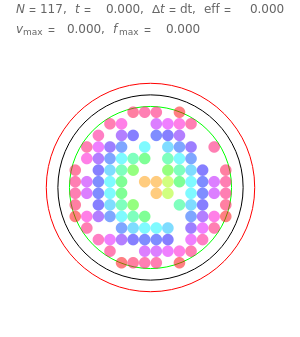
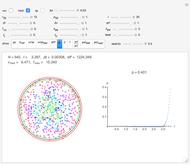
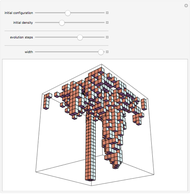
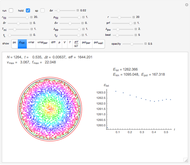
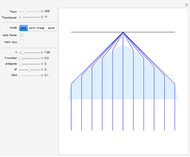
Snapshot 1: This is the system with the largest number of particles possible for this Demonstration. From the regularly packed initial configuration, a few integration steps were done. Notice the efficiency measure 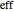 which, when compared with snapshots with fewer particles
which, when compared with snapshots with fewer particles 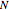 , shows that the actual computation time per integration step grows even more slowly than
, shows that the actual computation time per integration step grows even more slowly than 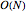 .
.
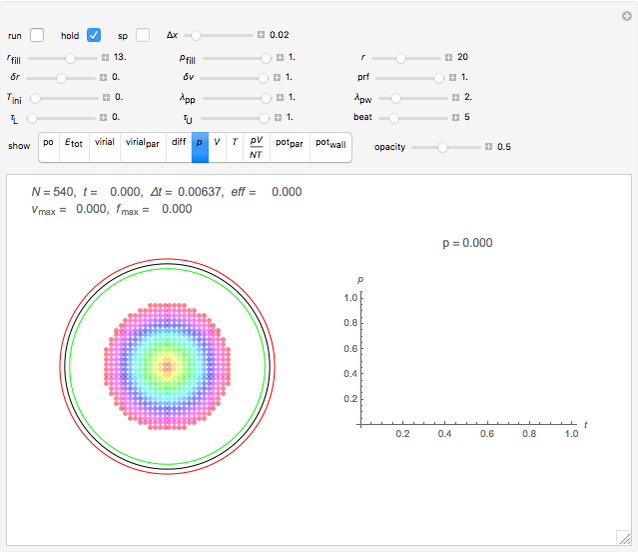
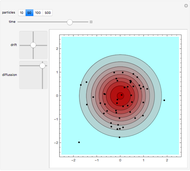
Snapshot 2: initial state of a compact system that will be heated and will evaporate as seen in the next snapshot
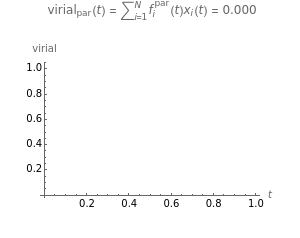
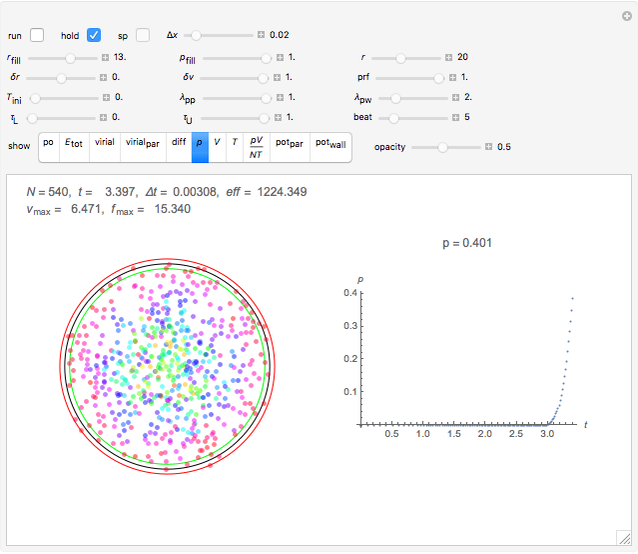
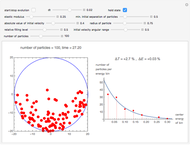
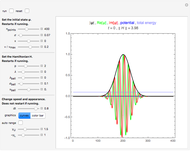
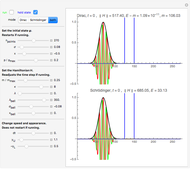
Snapshot 3: the pressure diagram shows that after some heating, particles arrive at the wall and exert forces on it
Snapshot 4: this shows the temperature growth during heating for the process shown in Snapshot 3
Snapshot 5: typical pressure fluctuations
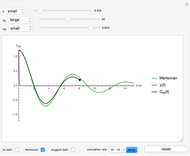
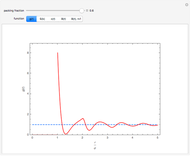
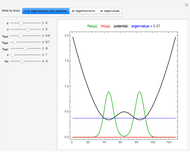
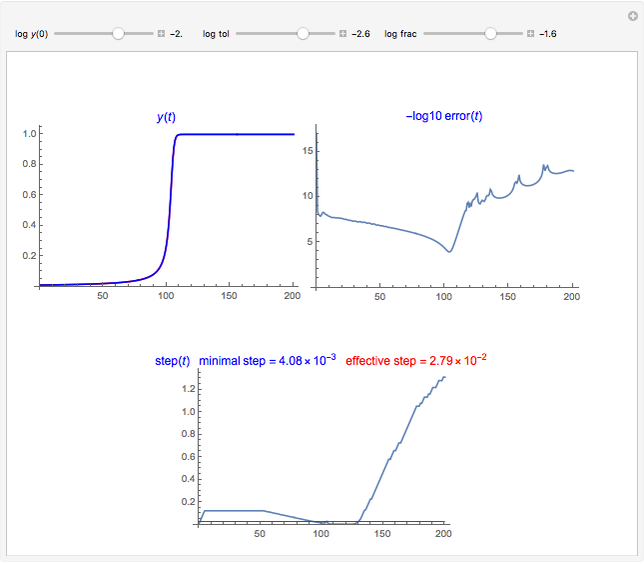
Snapshot 6: Deviation from the ideal gas equation of state for small particle-particle interaction. According to the ideal gas state equation (with the units used here and in 2D) ,the quantity shown here should oscillate around the value 1. The repulsive particle interaction shifts this to values larger than 1.
Snapshot 7: deviation from the ideal gas equation of state for 10-times-stronger particle-particle interaction
Snapshot 8: Condensation by cooling of binding particles in a wall-only container (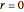 ). There are 25 particles that are distributed over nine positions.
). There are 25 particles that are distributed over nine positions.
Permanent Citation