Stripping Column Operation

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
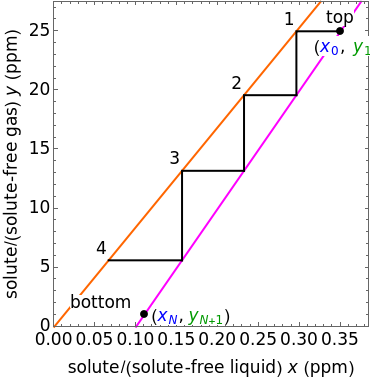
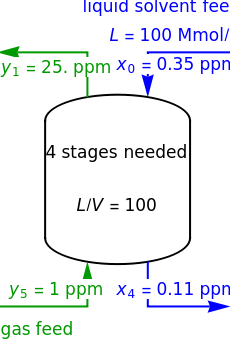
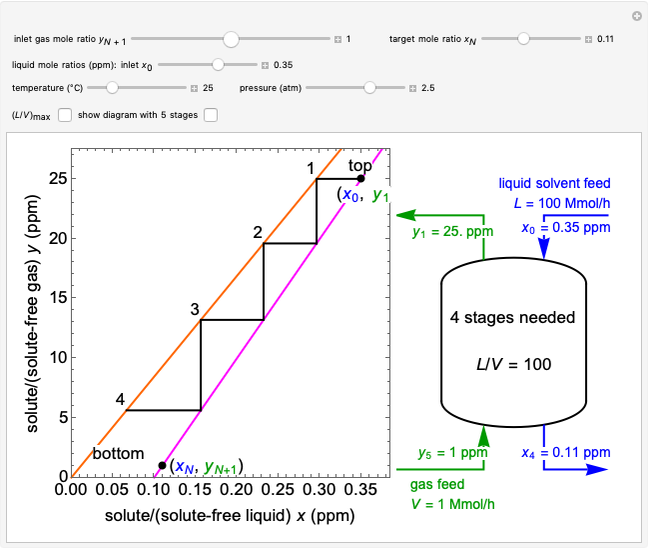
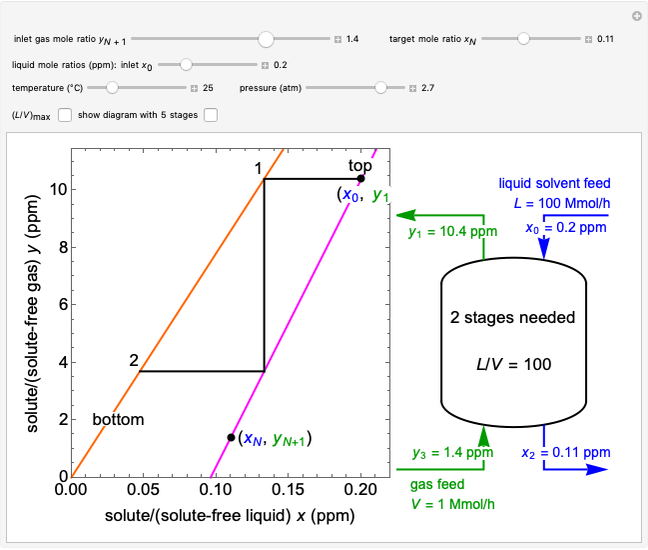
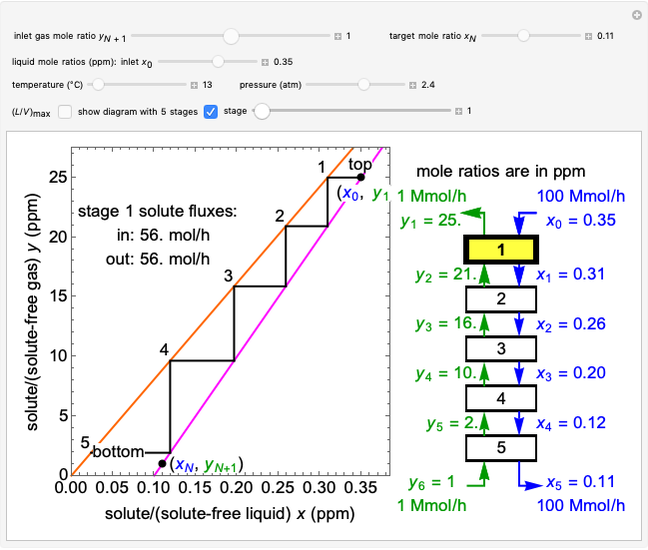
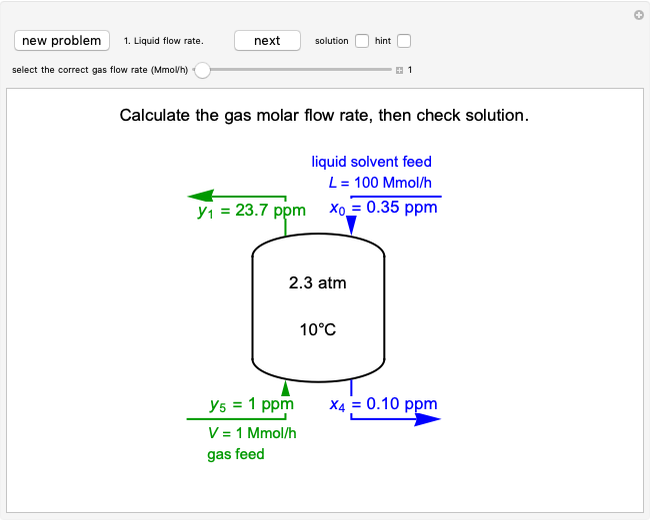
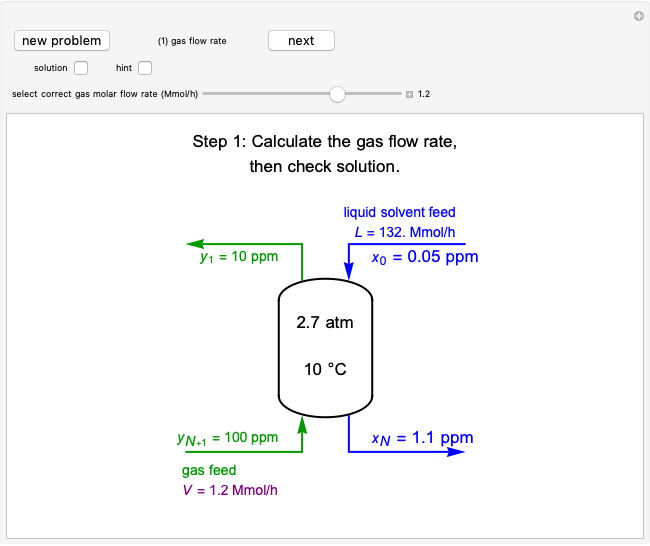
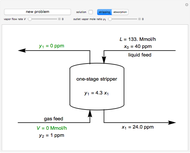
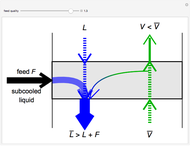
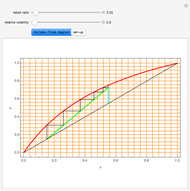
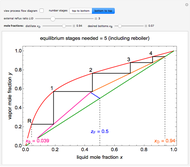
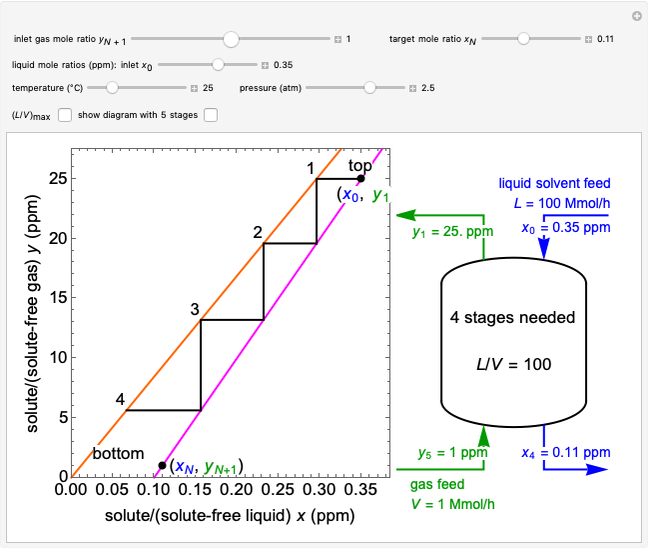
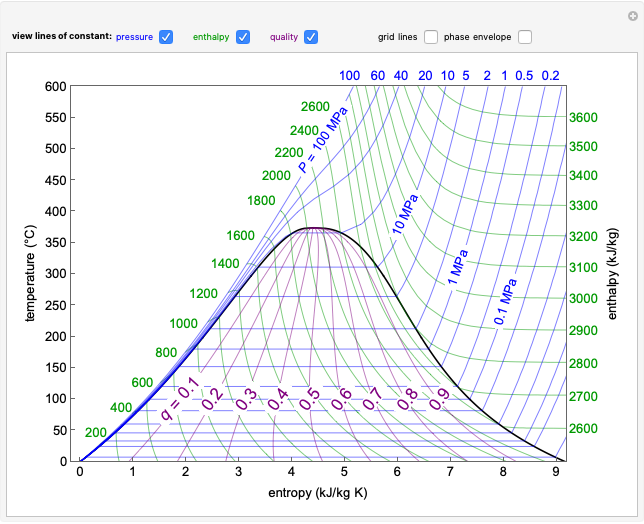
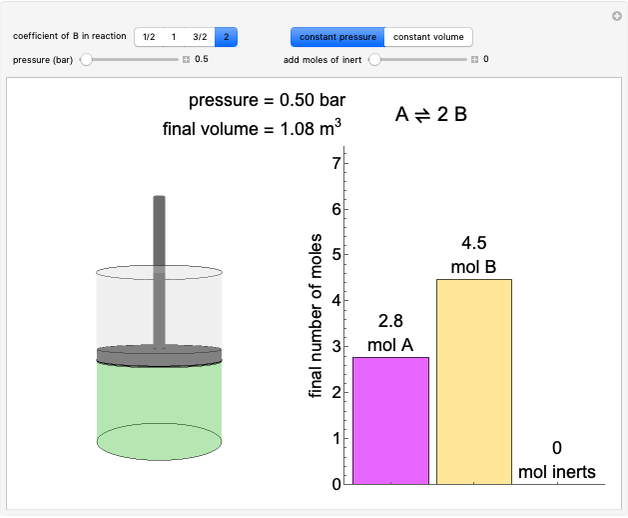
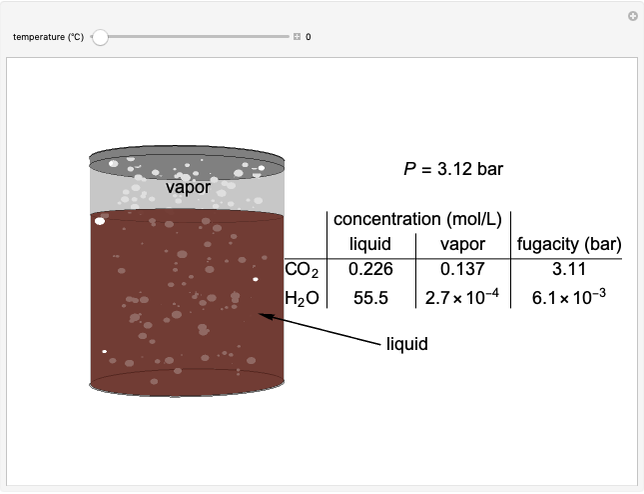
In this Demonstration, a trayed stripping column is used to remove an impurity from a liquid feed by stripping the impurity into a gas stream. The pink operating line is obtained from a mass balance, and its slope 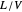 (the ratio of liquid flow rate to gas flow rate) is shown on the column on the right. The phase equilibrium line, which is obtained from Henry's law, is orange. The top and bottom of the column are labeled on the
(the ratio of liquid flow rate to gas flow rate) is shown on the column on the right. The phase equilibrium line, which is obtained from Henry's law, is orange. The top and bottom of the column are labeled on the  -
- diagram. Move your mouse over the pink and orange lines to see their labels. The number of trays/stages needed to obtain an outlet solute mole ratio of
diagram. Move your mouse over the pink and orange lines to see their labels. The number of trays/stages needed to obtain an outlet solute mole ratio of 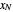 ppm in the liquid stream is calculated. A stage is a plate that contacts the liquid solvent and the gas to promote mass transfer. When a partial stage is calculated, the number of stages is rounded down to the nearest full stage. Use the sliders to change the pressure and temperature in the column, the gas flow rate
ppm in the liquid stream is calculated. A stage is a plate that contacts the liquid solvent and the gas to promote mass transfer. When a partial stage is calculated, the number of stages is rounded down to the nearest full stage. Use the sliders to change the pressure and temperature in the column, the gas flow rate 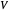 and the solute mole ratio in the gas feed,
and the solute mole ratio in the gas feed, 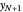 . Check the "
. Check the "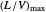 " box to show the maximum slope for the operating line; this condition would require an infinite number of stages. Check "show diagram with 5 stages" to set conditions that require five stages and display the mole ratios entering and leaving each stage. Use the "stage" slider to move through each stage and display the solute fluxes in and out of that stage on the
" box to show the maximum slope for the operating line; this condition would require an infinite number of stages. Check "show diagram with 5 stages" to set conditions that require five stages and display the mole ratios entering and leaving each stage. Use the "stage" slider to move through each stage and display the solute fluxes in and out of that stage on the  -
- diagram.
diagram.
Contributed by: Rachael L. Baumann (May 2018)
Additional contributions by: John L. Falconer
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
The equilibrium line is calculated using Henry's law:
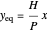 ,
,
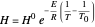 ,
,
where 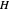 is Henry's constant (atm),
is Henry's constant (atm), 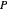 is pressure (atm),
is pressure (atm), 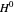 is Henry's constant at
is Henry's constant at 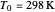 (atm),
(atm), 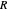 is the ideal gas constant (J/(mol K)) and
is the ideal gas constant (J/(mol K)) and 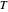 is temperature (K).
is temperature (K).
The operating line is calculated from a mass balance around the stripper:
 ,
,
which rearranges to:
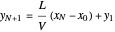 .
.
The operating line is then:
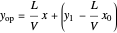 ,
,
where 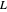 is the liquid solvent molar flow rate (Mmol/h),
is the liquid solvent molar flow rate (Mmol/h), 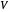 is the gas molar flow rate (Mmol/h),
is the gas molar flow rate (Mmol/h), 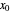 is the mole ratio of the impurity in the inlet liquid solvent stream (ppm),
is the mole ratio of the impurity in the inlet liquid solvent stream (ppm), 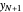 is the mole ratio of the impurity in the inlet gas stream (ppm),
is the mole ratio of the impurity in the inlet gas stream (ppm), 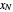 is the mole ratio of the impurity in the outlet liquid solvent stream (ppm) and
is the mole ratio of the impurity in the outlet liquid solvent stream (ppm) and 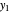 is the mole ratio of the impurity in the outlet gas stream (ppm).
is the mole ratio of the impurity in the outlet gas stream (ppm).
To count off stages, start at 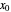 on the operating line
on the operating line 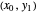 , then draw a horizontal line to the equilibrium line
, then draw a horizontal line to the equilibrium line 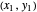 . Then draw a vertical line down to the operating line. Repeat these steps until
. Then draw a vertical line down to the operating line. Repeat these steps until 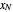 is reached.
is reached.
The outlet liquid mole ratio 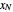 is calculated from the mass balance:
is calculated from the mass balance:
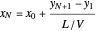 .
.
Reference
[1] P. C. Wankat, Separation Process Engineering: Includes Mass Transfer Analysis, 3rd ed., Upper Saddle River, NJ: Prentice Hall, 2011.
Permanent Citation