The Schnakenberg Reaction

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
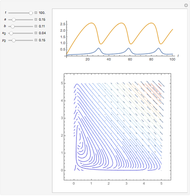
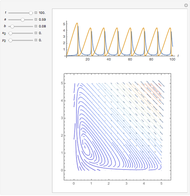
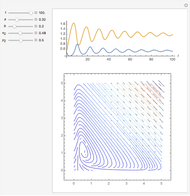
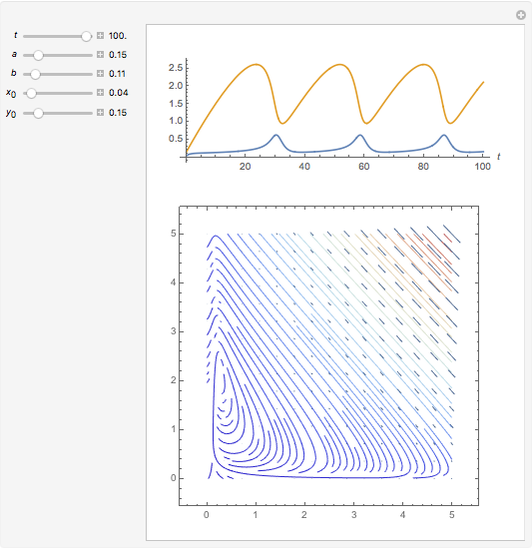
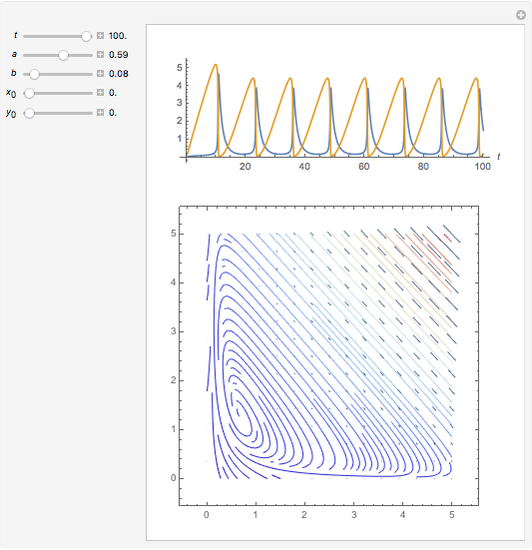
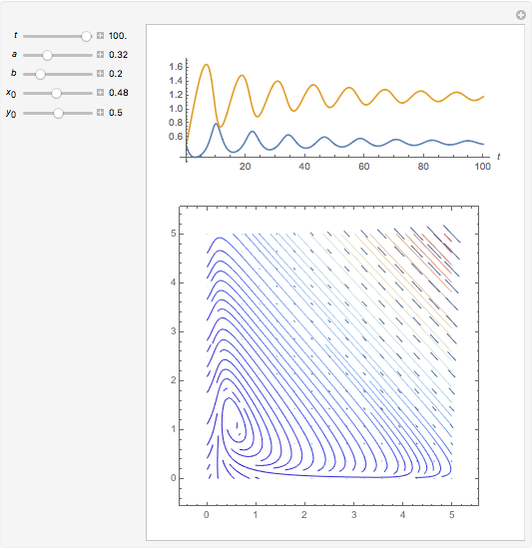
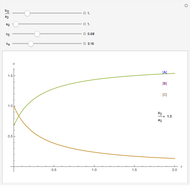
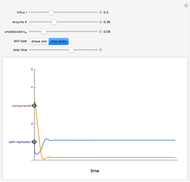
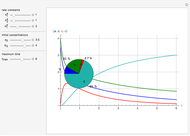
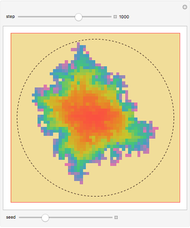
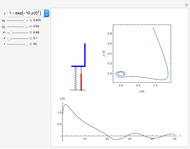
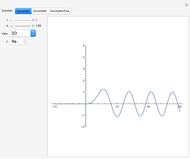
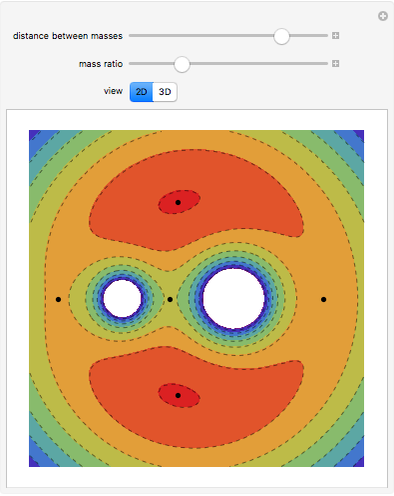
In 1979, J. Schnakenberg introduced a system showing sustained oscillations for a simple model of glycolysis (a metabolic process that converts glucose to provide energy for metabolism), very similar to a system known as the "Brusselator" that consists of four reactions.
Contributed by: Enrique Zeleny (January 2013)
Open content licensed under CC BY-NC-SA
Snapshots
Details
The reaction is trimolecular and involves autocatalysis: the reaction product is itself a catalyst for one step of the reaction. The reaction proceeds in three steps:
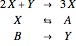
following the kinetic equations
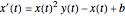 ,
,
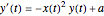 .
.
Reference
[1] J. Schnakenberg, "Simple Chemical Reaction Systems with Limit Cycle Behaviour," Journal of Theoretical Biology, 81(3), 1979 pp. 389–400. doi: 10.1016/0022-5193(79)90042-0.
Permanent Citation