An optimized three-parameter variational wavefunction for the helium isoelectronic series  ,
,  ,
,  , …,
, …,  can give quite accurate values for the ground-state energies. This computation is based on a simplified version of Hylleraas' classic work on two-electron atoms [1]. The Schrödinger equation for a two-electron atom with nuclear charge
can give quite accurate values for the ground-state energies. This computation is based on a simplified version of Hylleraas' classic work on two-electron atoms [1]. The Schrödinger equation for a two-electron atom with nuclear charge  can be written as
can be written as
 ,
,
with the Hamiltonian

expressed in atomic units  . For S-states, including the ground state, the wavefunction depends on just three variables:
. For S-states, including the ground state, the wavefunction depends on just three variables:  ,
,  , and
, and  . Hylleraas used these alternative variables:
. Hylleraas used these alternative variables:  ,
,  , and
, and  . Consider simple trial functions of the form
. Consider simple trial functions of the form  . We need the combination
. We need the combination  in order that
in order that  is an even function of
is an even function of  , thus a symmetric function with respect to the interchange of
, thus a symmetric function with respect to the interchange of  and
and  .
.
According to the variational principle, the ground-state energy can be approximated by  , where
, where

 .
.
The parameters  ,
,  , and
, and  are then selected such that
are then selected such that  takes a minimum value. This is always an upper bound on the exact ground-state energy
takes a minimum value. This is always an upper bound on the exact ground-state energy  . To run this Demonstration, choose a value of
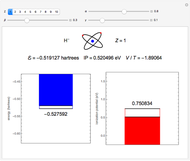
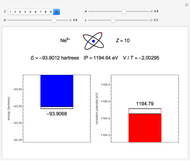
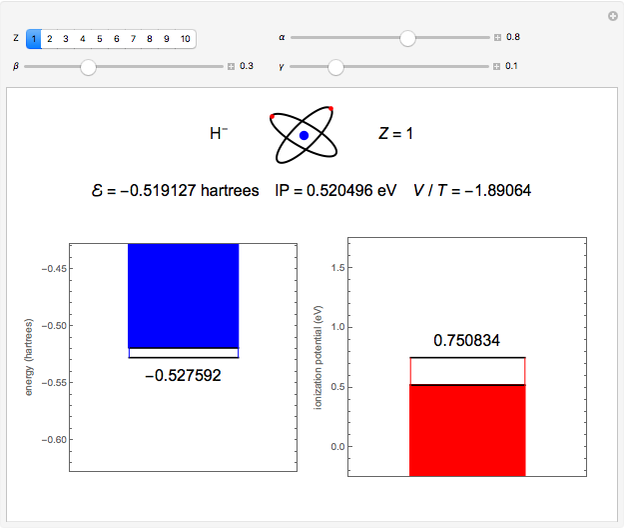
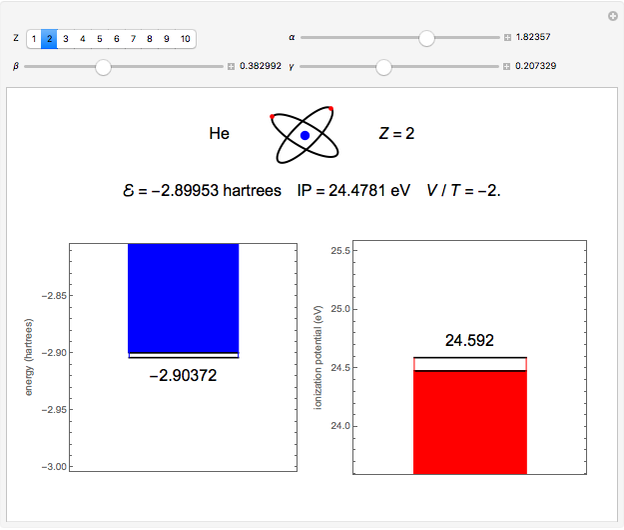
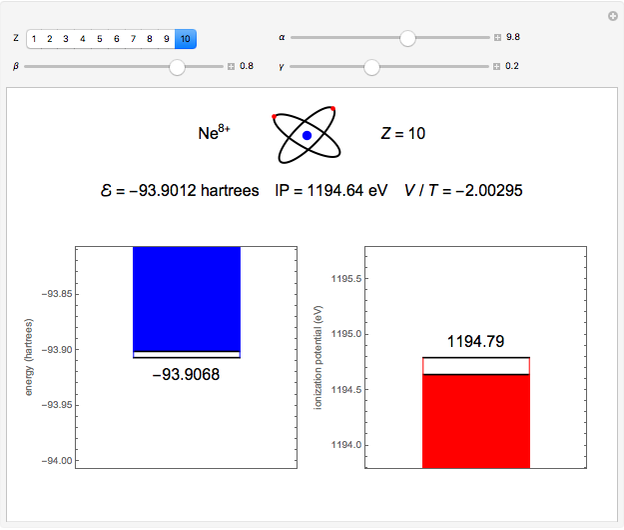
. To run this Demonstration, choose a value of  corresponding to one of the two-electron atoms or ions. Then, vary the sliders determining various combinations of α, β, and γ. The ionization potential (
corresponding to one of the two-electron atoms or ions. Then, vary the sliders determining various combinations of α, β, and γ. The ionization potential ( ) is the energy to remove one electron, which leaves the atom in the one-electron energy state
) is the energy to remove one electron, which leaves the atom in the one-electron energy state  . The
. The  is expressed in
is expressed in  (
( ). As the blue and red areas become larger, exact values of the energy and ionization potential are being approached more closely. Also shown is the ratio of expectation values of potential and kinetic energies,
). As the blue and red areas become larger, exact values of the energy and ionization potential are being approached more closely. Also shown is the ratio of expectation values of potential and kinetic energies,  . By the virial theorem for Coulombic systems, this ratio should equal
. By the virial theorem for Coulombic systems, this ratio should equal  . This value would be obtained by the exact solution of the Schrödinger equation, but also for an approximate solution with optimally chosen variational parameters.
. This value would be obtained by the exact solution of the Schrödinger equation, but also for an approximate solution with optimally chosen variational parameters.
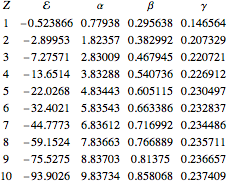
If you want to look at the answers, the optimal values of  ,
,  , and
, and  are tabulated in the Details section.
are tabulated in the Details section.
[less]

 ,
,  ,
,  , …,
, …,  can give quite accurate values for the ground-state energies. This computation is based on a simplified version of Hylleraas' classic work on two-electron atoms [1]. The Schrödinger equation for a two-electron atom with nuclear charge
can give quite accurate values for the ground-state energies. This computation is based on a simplified version of Hylleraas' classic work on two-electron atoms [1]. The Schrödinger equation for a two-electron atom with nuclear charge  can be written as
can be written as