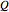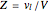Heating Water in a Closed Vessel
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
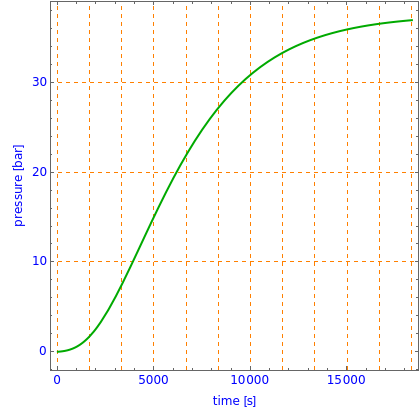
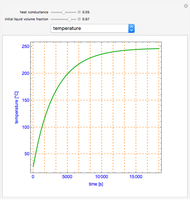
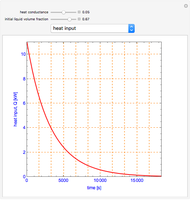
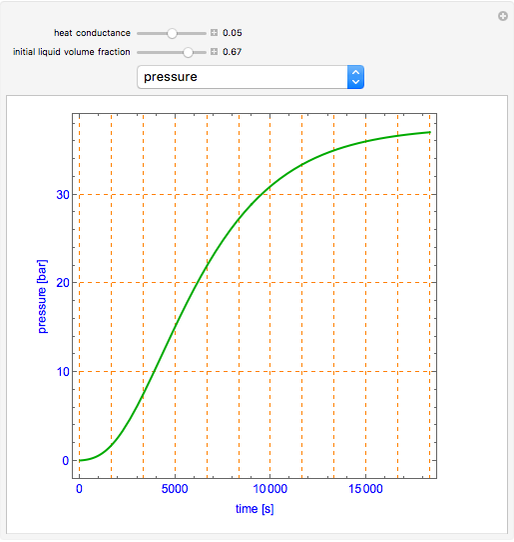
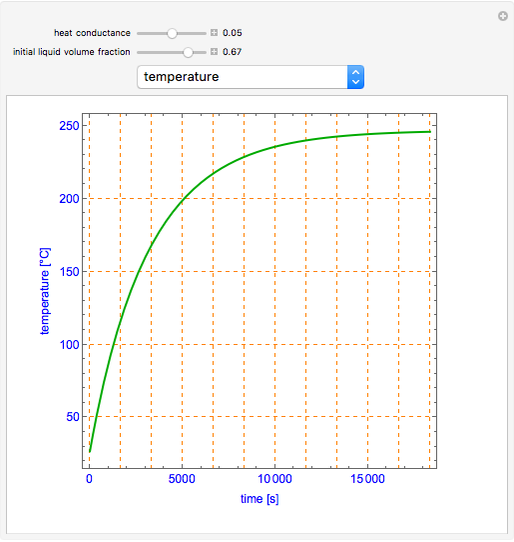
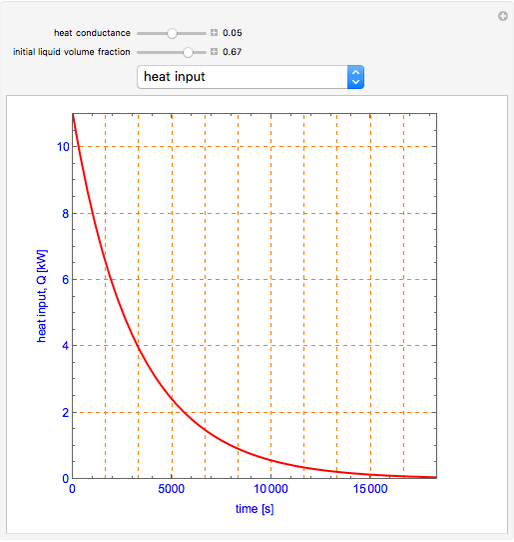
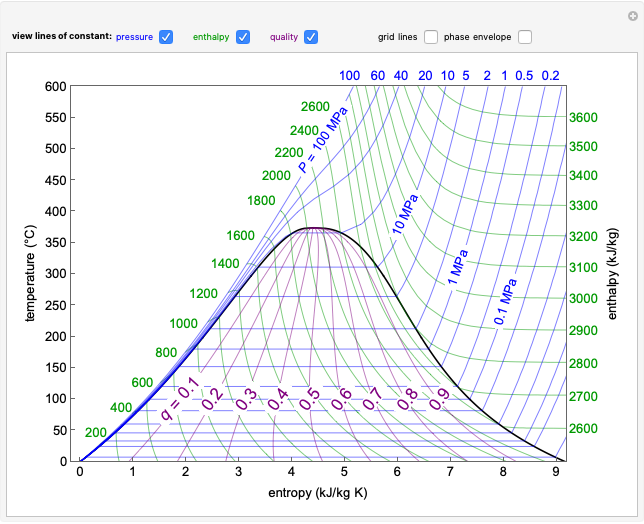
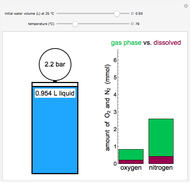
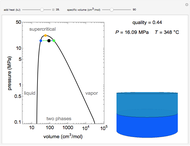
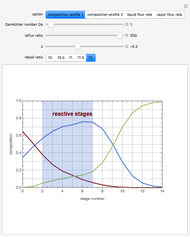
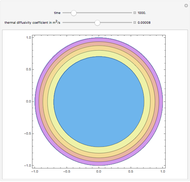
This Demonstration shows what happens to water, initially in liquid-vapor equilibrium at 300 K, if it is heated in a closed vessel with a given volume 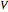 . The heating medium is saturated steam at 520 K and the initial liquid volume fraction is
. The heating medium is saturated steam at 520 K and the initial liquid volume fraction is 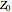 .
.
Contributed by: Housam Binous, Ismail Boukholda, and Ahmed Bellagi (February 2013)
Open content licensed under CC BY-NC-SA
Snapshots
Details
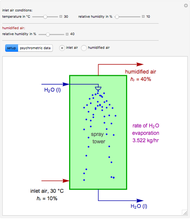
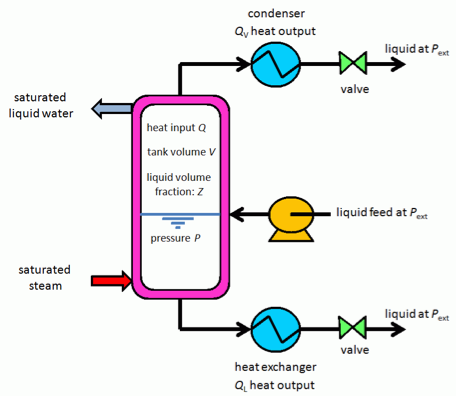
This is the experimental setup.
Permanent Citation