Steam Distillation of a Mixture of Hydrocarbons
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
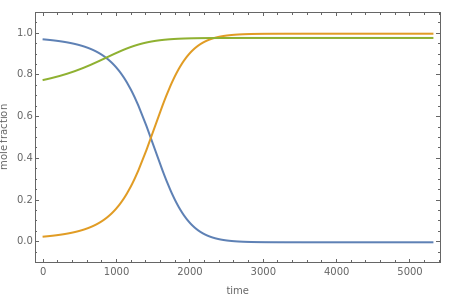
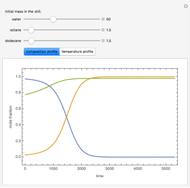
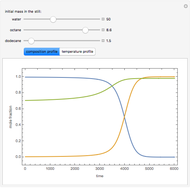
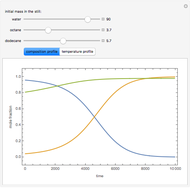
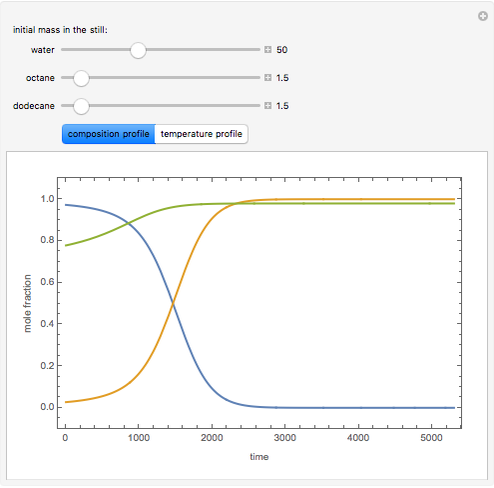
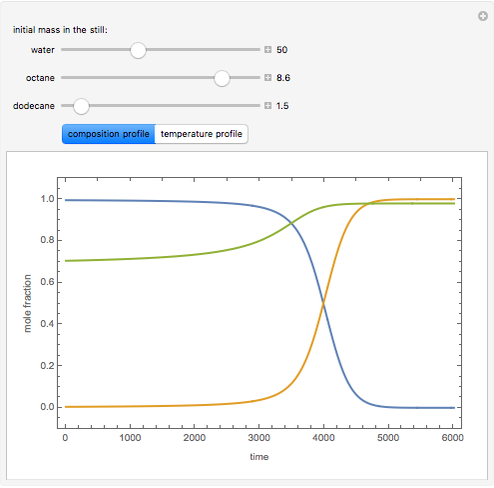
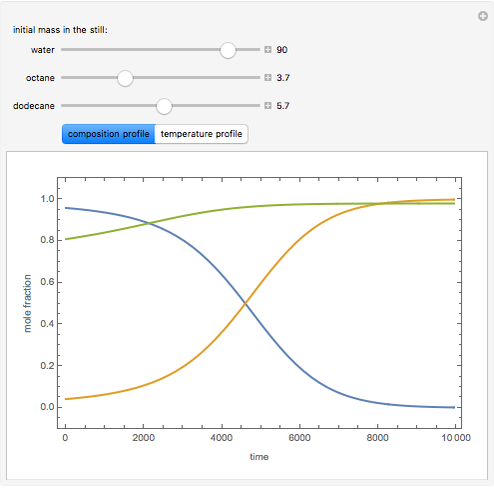
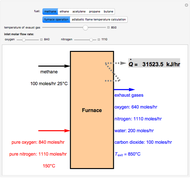
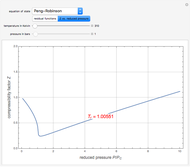
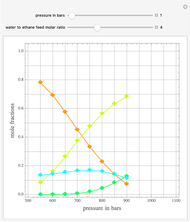
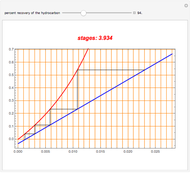
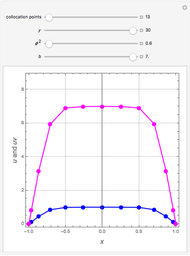
Consider a binary mixture of the hydrocarbons  -octane and
-octane and  -dodecane. The normal boiling point of this mixture is well above 100° C. However, if one adds water to the mixture, it is possible to separate the hydrocarbons at temperatures below 100° C by a method known as steam distillation.
-dodecane. The normal boiling point of this mixture is well above 100° C. However, if one adds water to the mixture, it is possible to separate the hydrocarbons at temperatures below 100° C by a method known as steam distillation.
Contributed by: Housam Binous (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Permanent Citation