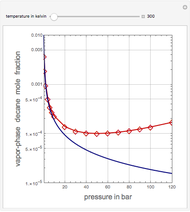
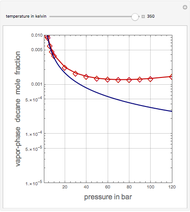
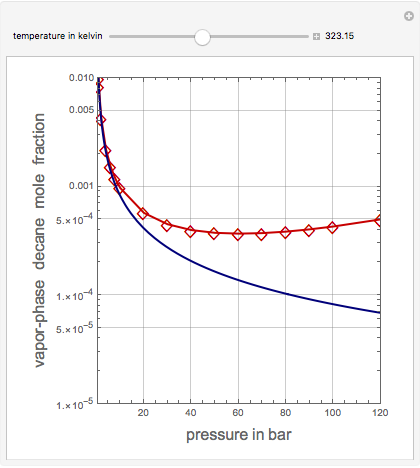
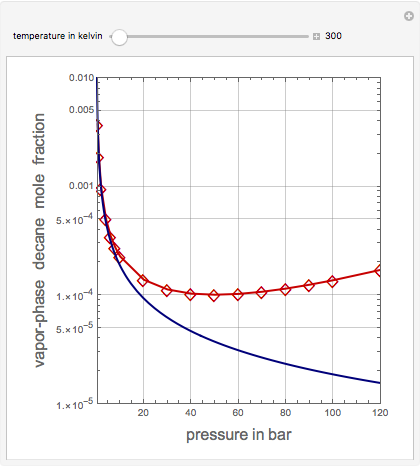
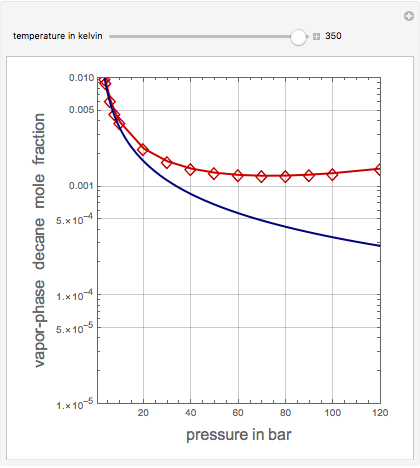
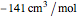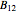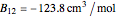Vapor-Phase Solubility of Decane in Nitrogen as Functions of Temperature and Pressure

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
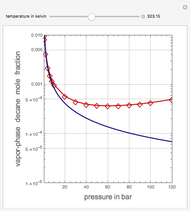
This Demonstration computes the solubility of a high-temperature boiling fluid ( -decane) in supercritical nitrogen at various high pressures for user-set values of the temperature. Two approximate methods for solubility prediction are compared: (1) the ideal gas assumption (blue curve); and (2) real gas behavior, described by the virial equation of state (red curve). Here, the fugacity coefficients are computed using the virial equation.
-decane) in supercritical nitrogen at various high pressures for user-set values of the temperature. Two approximate methods for solubility prediction are compared: (1) the ideal gas assumption (blue curve); and (2) real gas behavior, described by the virial equation of state (red curve). Here, the fugacity coefficients are computed using the virial equation.
Contributed by: Housam Binous, Brian G. Higgins, Nadhir A. Al-Baghli, and Ahmed Bellagi (January 2013)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference
[1] J. M. Prausnitz, R. N. Lichtenthaler, and E. Gomes de Azevedo, Molecular Thermodynamics of Fluid-Phase Equilibria, 3rd ed., Upper Saddle River, NJ: Prentice Hall, 1999.
Permanent Citation