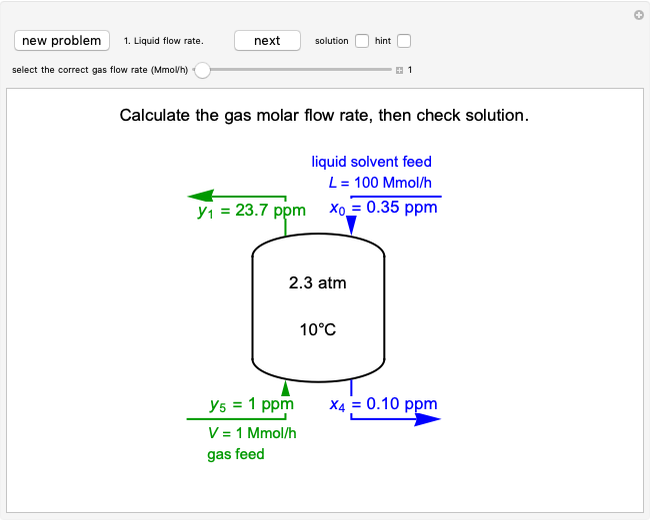
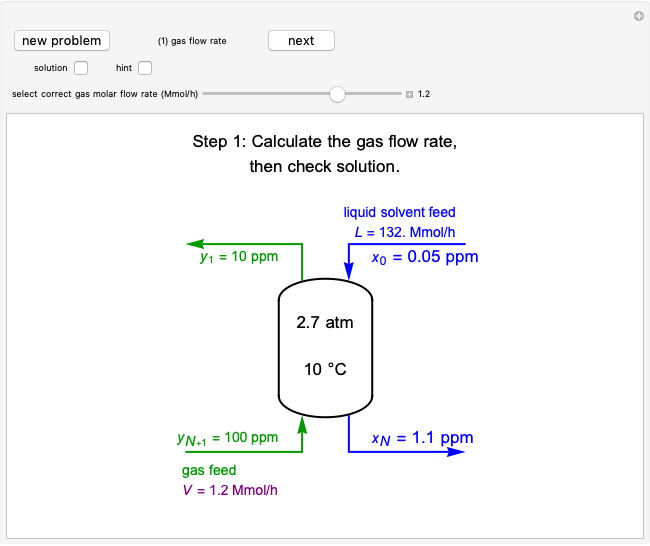
Temperature Changes in an Ideal Gas

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
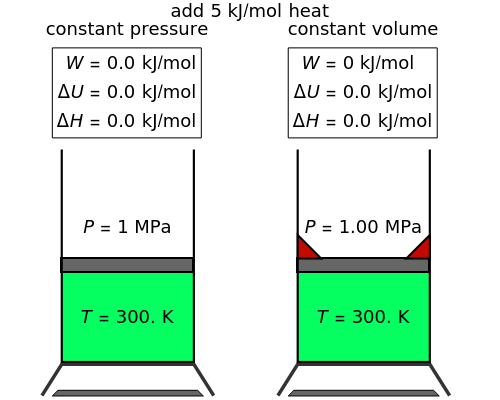
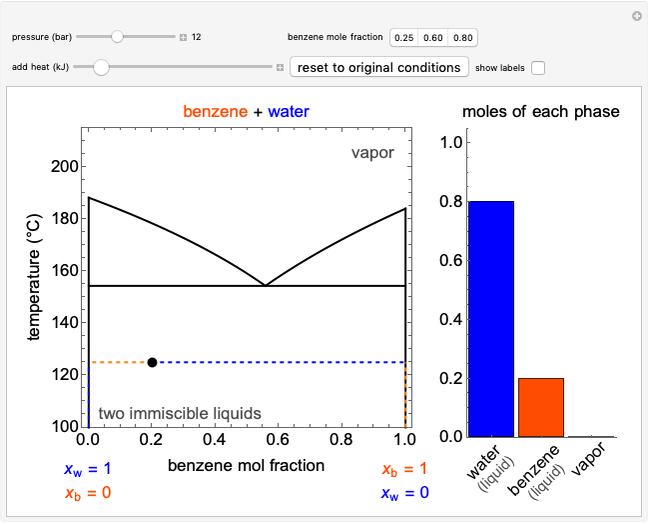
In this Demonstration, an ideal gas is heated or cooled in two containers, one at constant pressure and one at constant volume. You can vary the amount of heat added or removed with the slider. After setting "heat gas" or "cool gas", click the play button to the right of "add heat" or "remove heat" to initiate heat transfer. The first law and the ideal gas law are used to calculate the final temperature  and the changes in internal energy (
and the changes in internal energy ( ) and enthalpy (
) and enthalpy ( ) for each system. The work
) for each system. The work  is calculated for the constant-pressure process, and the final pressure
is calculated for the constant-pressure process, and the final pressure  is calculated for the constant-volume system.
is calculated for the constant-volume system.
Contributed by: Rachael L. Baumann (June 2015)
Additional contributions by: John L. Falconer
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
The final temperatures  of the constant-pressure and constant-volume systems are found using:
of the constant-pressure and constant-volume systems are found using:
 ,
,
where  is the change in internal energy,
is the change in internal energy,  is the heat added or removed and
is the heat added or removed and  is work, all in kJ/mol.
is work, all in kJ/mol.
For the constant-volume process:  .
.
For the constant-pressure process:  or
or  ,
,
so  ,
,
and so 
 for a constant-pressure process.
for a constant-pressure process.
Here  is pressure (Pa),
is pressure (Pa),  is molar volume (
is molar volume ( ),
),  is the ideal gas constant,
is the ideal gas constant,  is the temperature in K and
is the temperature in K and  is the enthalpy change (kJ/mol).
is the enthalpy change (kJ/mol).
For both processes:
 ,
,
 ,
,
 ,
,
 ,
,
where  and
and  are the constant-volume and constant-pressure heat capacities (kJ/[mol K]).
are the constant-volume and constant-pressure heat capacities (kJ/[mol K]).
The ideal gas law is used to calculate the final volume for the constant-pressure process and the final pressure for the constant-volume process:
 for constant pressure,
for constant pressure,
 for constant volume.
for constant volume.
The screencast video at [1] explains how to use this Demonstration.
Reference
[1] Temperature Changes in an Ideal Gas [Video]. (Sep 1, 2016) www.colorado.edu/learncheme/thermodynamics/TemperatureChangesinIdealGas.xhtml.
Permanent Citation